Genetic Variant in CHRNA5 and Response to Varenicline and Combination Nicotine Replacement in a Randomized Placebo-Controlled Trial
- PMID: 32602170
- PMCID: PMC7993015
- DOI: 10.1002/cpt.1971
Genetic Variant in CHRNA5 and Response to Varenicline and Combination Nicotine Replacement in a Randomized Placebo-Controlled Trial
Abstract
It is unclear if genetic variants affect smoking cessation treatment response. This study tested whether variants in the cholinergic receptor nicotinic alpha 5 subunit (CHRNA5) predict response to smoking cessation medication by directly comparing the two most effective smoking cessation pharmacotherapies. In this genotype-stratified randomized, double-blind, placebo-controlled clinical trial (May 2015-August 2019 in St Louis, Missouri), smokers were randomized by genotype in blocks of six (1:1:1 ratio) to three conditions: 12 weeks of placebo (n = 273), combination nicotine patch and lozenge (combination nicotine replacement therapy, cNRT, n = 275), or varenicline (n = 274). All participants received counseling and were followed for 12 months. The primary end point was biochemically verified 7-day point prevalence abstinence at the end of treatment (EOT, week 12). Trial registration and eligibility criteria are on clinicaltrials.gov (https://clinicaltrials.gov/) (NCT02351167). We conducted the genetic analyses separately for 516 European ancestry (EA) smokers and 306 non-EA smokers (including 270 African American smokers). In African American smokers, there was a genotype-by-treatment interaction for EOT abstinence (χ2 = 10.7, degrees of freedom = 2. P = 0.0049): specifically, cNRT was more effective in smokers with rs16969968 GG genotype than was placebo, while varenicline was more effective in smokers of GA/AA genotypes. In EA ancestry smokers, there was no significant genotype-by-treatment interaction. In the whole sample, although both were effective at EOT, only varenicline, and not cNRT, was significantly effective relative to placebo at 6-month follow-up. Importantly, this study suggests that genetic information can further enhance smoking cessation treatment effectiveness.
© 2020 The Authors Clinical Pharmacology & Therapeutics © 2020 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
CONFLICT OF INTEREST
All other authors declared no competing interests for this work.
Figures



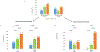

References
-
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 2014).
-
- Anthenelli RM et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 387, 2507–20 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

