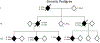
Longitudinal clinical, neuropsychological, and neuroimaging characterization of a kindred with a 12-octapeptide repeat insertion in PRNP: the next generation
- PMID: 32602775
- PMCID: PMC7426006
- DOI: 10.1080/13554794.2020.1787458
Longitudinal clinical, neuropsychological, and neuroimaging characterization of a kindred with a 12-octapeptide repeat insertion in PRNP: the next generation
Abstract
Background: Highly penetrant inherited mutations in the prion protein gene (PRNP) offer a window to study the pathobiology of prion disorders.
Method: Clinical, neuropsychological, and neuroimaging characterization of a kindred.
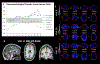
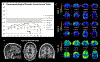
Results: Three of four mutation carriers have progressed to a frontotemporal dementia phenotype. Declines in neuropsychological function coincided with changes in FDG-PET at the identified onset of cognitive impairment.
Conclusions and relevance: Gene silencing treatments are on the horizon and when they become available, early detection will be crucial. Longitudinal studies involving familial mutation kindreds can offer important insights into the initial neuropsychological and neuroimaging changes necessary for early detection.
Keywords: FDG-PET; Genetic prion; neuroimaging; neuropsychological; octapeptide repeat insertion.
Figures


Similar articles
-
Octapeptide repeat alteration mutations of the prion protein gene in clinically diagnosed Alzheimer's disease and frontotemporal dementia.Clin Genet. 2023 Sep;104(3):350-355. doi: 10.1111/cge.14354. Epub 2023 May 6. Clin Genet. 2023. PMID: 37148197
-
Clinical characterization of a kindred with a novel 12-octapeptide repeat insertion in the prion protein gene.Arch Neurol. 2011 Sep;68(9):1165-70. doi: 10.1001/archneurol.2011.187. Arch Neurol. 2011. PMID: 21911696 Free PMC article.
-
MRS in early and presymptomatic carriers of a novel octapeptide repeat insertion in the prion protein gene.J Neuroimaging. 2013 Jul;23(3):409-13. doi: 10.1111/j.1552-6569.2012.00717.x. Epub 2012 May 21. J Neuroimaging. 2013. PMID: 22612156 Free PMC article.
-
Mutations in Prion Protein Gene: Pathogenic Mechanisms in C-Terminal vs. N-Terminal Domain, a Review.Int J Mol Sci. 2019 Jul 23;20(14):3606. doi: 10.3390/ijms20143606. Int J Mol Sci. 2019. PMID: 31340582 Free PMC article. Review.
-
Prion disease.Handb Clin Neurol. 2018;148:441-464. doi: 10.1016/B978-0-444-64076-5.00029-6. Handb Clin Neurol. 2018. PMID: 29478593 Review.
Cited by
-
Pooled analysis of patients with inherited prion disease caused by two- to twelve-octapeptide repeat insertions in the prion protein gene (PRNP).J Neurol. 2024 Jan;271(1):263-273. doi: 10.1007/s00415-023-11968-9. Epub 2023 Sep 9. J Neurol. 2024. PMID: 37689591
-
Alterations of Striatal Subregions in a Prion Protein Gene V180I Mutation Carrier Presented as Frontotemporal Dementia With Parkinsonism.Front Aging Neurosci. 2022 Apr 15;14:830602. doi: 10.3389/fnagi.2022.830602. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35493933 Free PMC article.
References
-
- Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, … Gorno-Tempini ML (2009). Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proc Natl Acad Sci U S A, 106(6), 2018–2022. doi:10.1073/pnas.0812697106 - DOI - PMC - PubMed
-
- Ballatore C, Lee VM-Y, & Trojanowski JQ (2007). Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature Reviews Neuroscience, 8(9), 663. - PubMed
-
- Benton AL, Sivan AB, Hamsher K. d., Varney NR, & Spreen O (1994). Contributions to neuropsychological assessment: A clinical manual: Oxford University Press, USA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
