Bat SARS-Like WIV1 coronavirus uses the ACE2 of multiple animal species as receptor and evades IFITM3 restriction via TMPRSS2 activation of membrane fusion
- PMID: 32602823
- PMCID: PMC7473123
- DOI: 10.1080/22221751.2020.1787797
Bat SARS-Like WIV1 coronavirus uses the ACE2 of multiple animal species as receptor and evades IFITM3 restriction via TMPRSS2 activation of membrane fusion
Abstract
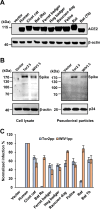
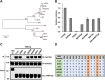
Diverse SARS-like coronaviruses (SL-CoVs) have been identified from bats and other animal species. Like SARS-CoV, some bat SL-CoVs, such as WIV1, also use angiotensin converting enzyme 2 (ACE2) from human and bat as entry receptor. However, whether these viruses can also use the ACE2 of other animal species as their receptor remains to be determined. We report herein that WIV1 has a broader tropism to ACE2 orthologs than SARS-CoV isolate Tor2. Among the 9 ACE2 orthologs examined, human ACE2 exhibited the highest efficiency to mediate the infection of WIV1 pseudotyped virus. Our findings thus imply that WIV1 has the potential to infect a wide range of wild animals and may directly jump to humans. We also showed that cell entry of WIV1 could be restricted by interferon-induced transmembrane proteins (IFITMs). However, WIV1 could exploit the airway protease TMPRSS2 to partially evade the IFITM3 restriction. Interestingly, we also found that amphotericin B could enhance the infectious entry of SARS-CoVs and SL-CoVs by evading IFITM3-mediated restriction. Collectively, our findings further underscore the risk of exposure to animal SL-CoVs and highlight the vulnerability of patients who take amphotericin B to infection by SL-CoVs, including the most recently emerging (SARS-CoV-2).
Keywords: ACE2 receptor; IFITM; SARS-like coronavirus WIV1; TMPRSS2; viral entry.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- WHO . Summary of probable SARS cases with onset of illness from November 1, 2002 to July 31, 2003 [cited 2004 Apr 21]; Available from: http://www.who.int/csr/sars/country/table2004_04_21/en/index.html). 2004.
-
- Li W, Shi Z, Yu M, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005 Oct 28;310(5748):676–679. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
