A Web-Based Mobile App (INTERACCT App) for Adolescents Undergoing Cancer and Hematopoietic Stem Cell Transplantation Aftercare to Improve the Quality of Medical Information for Clinicians: Observational Study
- PMID: 32602847
- PMCID: PMC7367529
- DOI: 10.2196/18781
A Web-Based Mobile App (INTERACCT App) for Adolescents Undergoing Cancer and Hematopoietic Stem Cell Transplantation Aftercare to Improve the Quality of Medical Information for Clinicians: Observational Study
Abstract
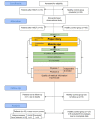
Background: A growing number of cancer and hematopoietic stem cell transplant (HSCT) survivors require long-term follow-up with optimal communication schemes, and patients' compliance is crucial. Adolescents have various unmet needs. Regarding self-report of symptoms and health status, users of mobile apps showed enhanced compliance. Currently, HSCT aftercare at the HSCT outpatient clinic of the St. Anna Children's Hospital in Vienna, Austria, is based on handwritten diaries, carrying various disadvantages. Recently, we developed the prototype of a web-based, self-monitoring gamified mobile app tailored for adolescents: the INTERACCT (Integrating Entertainment and Reaction Assessment into Child Cancer Therapy) app.
Objective: This observational, prospective study evaluated the usability of the INTERACCT app for tracking real-time self-reported symptoms and health status data in adolescent HSCT patients and a healthy matched control group. The primary outcome of the study was the quality of the self-reported medical information. We hypothesized that the mobile app would provide superior medical information for the clinicians than would the handwritten diaries.
Methods: Health data were reported via paper diary and mobile app for 5 consecutive days each. The quality of medical information was rated on a 5-point scale independently and blinded by two HSCT clinicians, and the duration of use was evaluated. A total of 52 participant questionnaires were assessed for gaming patterns and device preferences, self-efficacy, users' satisfaction, acceptability, and suggestions for improvement of the mobile app. Interrater reliability was calculated with the intraclass correlation coefficient, based on a two-way mixed model; one-way repeated-measures analysis of variance and t tests were conducted post hoc. Descriptive methods were used for correlation with participants' demographics. For users' satisfaction and acceptability of the mobile app, the median and the IQR were calculated.
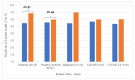
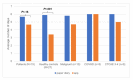
Results: Data from 42 participants-15 patients and 27 healthy students-with comparable demographics were evaluated. The results of our study indicated a superiority of the quality of self-reported medical data in the INTERACCT app over traditional paper-and-pencil assessment (mobile app: 4.14 points, vs paper-based diary: 3.77 points, P=.02). The mobile app outperformed paper-and-pencil assessments mainly among the patients, in particular among patients with treatment-associated complications (mobile app: 4.43 points, vs paper-based diary: 3.73 points, P=.01). The mobile app was used significantly longer by adolescents (≥14 years: 4.57 days, vs ≤13 years: 3.14 days, P=.03) and females (4.76 days for females vs 2.95 days for males, P=.004). This corresponds with a longer duration of use among impaired patients with comorbidities. User satisfaction and acceptability ratings for the mobile app were high across all groups, but adherence to entering a large amount of data decreased over time. Based on our results, we developed a case vignette of the target group.
Conclusions: Our study was the first to show that the quality of patient-reported medical information submitted via the INTERACCT app embedded in a serious game is superior to that submitted via a handwritten diary. In light of these results, a refinement of the mobile app supported by a machine learning approach is planned within an international research project.
Keywords: adolescents; cancer; medical information exchange; mobile app; mobile phone; self-reported heath status; stem cell transplant.
©Anita Lawitschka, Stephanie Buehrer, Dorothea Bauer, Konrad Peters, Marisa Silbernagl, Natalia Zubarovskaya, Barbara Brunmair, Fares Kayali, Helmut Hlavacs, Ruth Mateus-Berr, David Riedl, Gerhard Rumpold, Christina Peters. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 30.06.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
Supporting Medication Adherence in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplant Using the BMT4me mHealth App: Mixed Methods Usability Study.JMIR Cancer. 2025 May 29;11:e66847. doi: 10.2196/66847. JMIR Cancer. 2025. PMID: 40440561 Free PMC article.
-
Mobile Application vs Paper Pictorial Blood Assessment Chart to Track Menses in Young Women: A Randomized Cross-over Design.J Pediatr Adolesc Gynecol. 2018 Apr;31(2):84-88. doi: 10.1016/j.jpag.2017.09.009. Epub 2017 Oct 10. J Pediatr Adolesc Gynecol. 2018. PMID: 29030160 Clinical Trial.
-
Usability and User Experience of an mHealth App for Therapy Support of Patients With Breast Cancer: Mixed Methods Study Using Eye Tracking.JMIR Hum Factors. 2024 Mar 5;11:e50926. doi: 10.2196/50926. JMIR Hum Factors. 2024. PMID: 38441959 Free PMC article. Clinical Trial.
-
Mobile App-Based Self-Report Questionnaires for the Assessment and Monitoring of Bipolar Disorder: Systematic Review.JMIR Form Res. 2021 Jan 8;5(1):e13770. doi: 10.2196/13770. JMIR Form Res. 2021. PMID: 33416510 Free PMC article. Review.
-
Mobile Health Apps on COVID-19 Launched in the Early Days of the Pandemic: Content Analysis and Review.JMIR Mhealth Uhealth. 2020 Sep 16;8(9):e19796. doi: 10.2196/19796. JMIR Mhealth Uhealth. 2020. PMID: 32609622 Free PMC article. Review.
Cited by
-
Evaluating Simplified Web Interfaces of Risk Models for Clinical Use: Pilot Survey Study.JMIR Form Res. 2021 Jul 16;5(7):e22110. doi: 10.2196/22110. JMIR Form Res. 2021. PMID: 34269692 Free PMC article.
-
Investigating the Use of Serious Games for Cancer Control Among Children and Adolescents: Scoping Review.JMIR Serious Games. 2024 Jul 10;12:e58724. doi: 10.2196/58724. JMIR Serious Games. 2024. PMID: 38985502 Free PMC article.
-
Development and psychometric testing of a pediatric chronic graft-versus-host disease symptom scale: protocol for a two-phase, mixed methods study.Front Psychol. 2024 Jan 8;14:1243005. doi: 10.3389/fpsyg.2023.1243005. eCollection 2023. Front Psychol. 2024. PMID: 38259542 Free PMC article.
-
The most used questionnaires for evaluating satisfaction, usability, acceptance, and quality outcomes of mobile health.BMC Med Inform Decis Mak. 2022 Jan 27;22(1):22. doi: 10.1186/s12911-022-01764-2. BMC Med Inform Decis Mak. 2022. PMID: 35081953 Free PMC article. Review.
-
Conceptual Ambiguity Surrounding Gamification and Serious Games in Health Care: Literature Review and Development of Game-Based Intervention Reporting Guidelines (GAMING).J Med Internet Res. 2021 Sep 10;23(9):e30390. doi: 10.2196/30390. J Med Internet Res. 2021. PMID: 34505840 Free PMC article.
References
-
- Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: Health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant. 2012 Feb;18(2):162–171. doi: 10.1016/j.bbmt.2011.12.501. https://linkinghub.elsevier.com/retrieve/pii/S1083-8791(11)01063-9 - DOI - PMC - PubMed
-
- Fraser CJ, Bhatia S, Ness K, Carter A, Francisco L, Arora M, Parker P, Forman S, Weisdorf D, Gurney JG, Baker KS. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: A report from the Bone Marrow Transplant Survivor Study. Blood. 2006 Oct 15;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. http://europepmc.org/abstract/MED/16788100 - DOI - PMC - PubMed
-
- Sun C, Francisco L, Kawashima T, Leisenring W, Robison LL, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: A report from the Bone Marrow Transplant Survivor Study. Blood. 2010 Oct 28;116(17):3129–3139; quiz 3377. doi: 10.1182/blood-2009-06-229369. http://europepmc.org/abstract/MED/20656930 - DOI - PMC - PubMed
-
- Lawitschka A, Güclü ED, Varni JW, Putz M, Wolff D, Pavletic S, Greinix H, Peters C, Felder-Puig R. Health-related quality of life in pediatric patients after allogeneic SCT: Development of the PedsQL Stem Cell Transplant module and results of a pilot study. Bone Marrow Transplant. 2014 Aug;49(8):1093–1097. doi: 10.1038/bmt.2014.96. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

