Cryogenic OrbiSIMS Localizes Semi-Volatile Molecules in Biological Tissues
- PMID: 32603009
- PMCID: PMC7589292
- DOI: 10.1002/anie.202006881
Cryogenic OrbiSIMS Localizes Semi-Volatile Molecules in Biological Tissues
Abstract
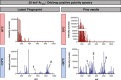
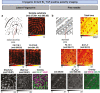
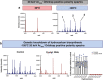
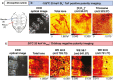
OrbiSIMS is a recently developed instrument for label-free imaging of chemicals with micron spatial resolution and high mass resolution. We report a cryogenic workflow for OrbiSIMS (Cryo-OrbiSIMS) that improves chemical detection of lipids and other biomolecules in tissues. Cryo-OrbiSIMS boosts ionization yield and decreases ion-beam induced fragmentation, greatly improving the detection of biomolecules such as triacylglycerides. It also increases chemical coverage to include molecules with intermediate or high vapor pressures, such as free fatty acids and semi-volatile organic compounds (SVOCs). We find that Cryo-OrbiSIMS reveals the hitherto unknown localization patterns of SVOCs with high spatial and chemical resolution in diverse plant, animal, and human tissues. We also show that Cryo-OrbiSIMS can be combined with genetic analysis to identify enzymes regulating SVOC metabolism. Cryo-OrbiSIMS is applicable to high resolution imaging of a wide variety of non-volatile and semi-volatile molecules across many areas of biomedicine.
Keywords: analytical methods; biological mass spectrometry imaging; lipids; semi-volatile molecules.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yew J. Y., Chung H., Prog. Lipid Res. 2015, 59, 88–105. - PubMed
-
- Xu H., Turlings T. C. J., Trends Plant Sci. 2018, 23, 100–111. - PubMed
-
- Lucattini L., Poma G., Covaci A., de Boer J., Lamoree M. H., Leonards P. E. G., Chemosphere 2018, 201, 466–482. - PubMed
-
- Vrkoslav V., Muck A., Cvačka J., Svatoš A., J. Am. Soc. Mass Spectrom. 2010, 21, 220–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

