Management of Breast Cancer During the COVID-19 Pandemic: A Stage- and Subtype-Specific Approach
- PMID: 32603252
- PMCID: PMC7564133
- DOI: 10.1200/OP.20.00364
Management of Breast Cancer During the COVID-19 Pandemic: A Stage- and Subtype-Specific Approach
Abstract
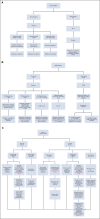
The COVID-19 pandemic has rapidly changed delivery of cancer care. Many nonurgent surgeries are delayed to preserve hospital resources, and patient visits to health care settings are limited to reduce exposure to SARS-CoV-2. Providers must carefully weigh risks and benefits of delivering immunosuppressive therapy during the pandemic. For breast cancer, a key difference is increased use of neoadjuvant systemic therapy due to deferral of many breast surgeries during the pandemic. In some cases, this necessitates increased use of genomic tumor profiling on core biopsy specimens to guide neoadjuvant therapy decisions. Breast cancer treatment during the pandemic requires multidisciplinary input and varies according to stage, tumor biology, comorbidities, age, patient preferences, and available hospital resources. We present here the Johns Hopkins Women's Malignancies Program approach to breast cancer management during the COVID-19 pandemic. We include algorithms based on tumor biology and extent of disease that guide management decisions during the pandemic. These algorithms emphasize medical oncology treatment decisions and demonstrate how we have operationalized the general treatment recommendations during the pandemic proposed by national groups, such as the COVID-19 Pandemic Breast Cancer Consortium. Our recommendations can be adapted by other institutions and medical oncology practices in accordance with local conditions and resources. Guidelines such as these will be important as we continue to balance treatment of breast cancer against risk of SARS-CoV-2 exposure and infection until approval of a vaccine.
Figures
References
-
- World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- Cannistra SA, Haffty BG, Ballman K. Challenges faced by medical journals during the COVID-19 pandemic. J Clin Oncol. 2020;38:2206–2207. - PubMed
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

