Sodium-calcium exchanger 1 is the key molecule for urinary potassium excretion against acute hyperkalemia
- PMID: 32603346
- PMCID: PMC7326190
- DOI: 10.1371/journal.pone.0235360
Sodium-calcium exchanger 1 is the key molecule for urinary potassium excretion against acute hyperkalemia
Abstract
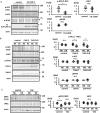
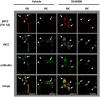
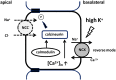
The sodium (Na+)-chloride cotransporter (NCC) expressed in the distal convoluted tubule (DCT) is a key molecule regulating urinary Na+ and potassium (K+) excretion. We previously reported that high-K+ load rapidly dephosphorylated NCC and promoted urinary K+ excretion in mouse kidneys. This effect was inhibited by calcineurin (CaN) and calmodulin inhibitors. However, the detailed mechanism through which high-K+ signal results in CaN activation remains unknown. We used Flp-In NCC HEK293 cells and mice to evaluate NCC phosphorylation. We analyzed intracellular Ca2+ concentration ([Ca2+]in) using live cell Ca2+ imaging in HEK293 cells. We confirmed that high-K+-induced NCC dephosphorylation was not observed without CaN using Flp-In NCC HEK29 cells. Extracellular Ca2+ reduction with a Ca2+ chelator inhibited high-K+-induced increase in [Ca2+]in and NCC dephosphorylation. We focused on Na+/Ca2+ exchanger (NCX) 1, a bidirectional regulator of cytosolic Ca2+ expressed in DCT. We identified that NCX1 suppression with a specific inhibitor (SEA0400) or siRNA knockdown inhibited K+-induced increase in [Ca2+]in and NCC dephosphorylation. In a mouse study, SEA0400 treatment inhibited K+-induced NCC dephosphorylation. SEA0400 reduced urinary K+ excretion and induced hyperkalemia. Here, we identified NCX1 as a key molecule in urinary K+ excretion promoted by CaN activation and NCC dephosphorylation in response to K+ load.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Riphagen IJ, Gijsbers L, van Gastel MD, Kema IP, Gansevoort RT, Navis G, et al. Effects of potassium supplementation on markers of osmoregulation and volume regulation: results of a fully controlled dietary intervention study. J Hypertens. 2016;34(2):215–20. 10.1097/HJH.0000000000000786 . - DOI - PubMed
-
- Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018;392(10146):496–506. Epub 2018/08/09. 10.1016/S0140-6736(18)31376-X . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

