CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity
- PMID: 32603414
- PMCID: PMC7685207
- DOI: 10.1182/blood.2020006200
CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity
Abstract
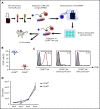
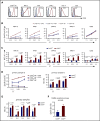
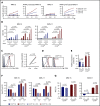
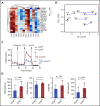
Multiple myeloma (MM) is a plasma cell neoplasm that commonly expresses CD38. Daratumumab (DARA), a human monoclonal antibody targeting CD38, has significantly improved the outcome of patients with relapsed or refractory MM, but the response is transient in most cases. Putative mechanisms of suboptimal efficacy of DARA include downregulation of CD38 expression and overexpression of complement inhibitory proteins on MM target cells as well as DARA-induced depletion of CD38high natural killer (NK) cells resulting in crippled antibody-dependent cellular cytotoxicity (ADCC). Here, we tested whether maintaining NK cell function during DARA therapy could maximize DARA-mediated ADCC against MM cells and deepen the response. We used the CRISPR/Cas9 system to delete CD38 (CD38KO) in ex vivo expanded peripheral blood NK cells. These CD38KO NK cells were completely resistant to DARA-induced fratricide, showed superior persistence in immune-deficient mice pretreated with DARA, and enhanced ADCC activity against CD38-expressing MM cell lines and primary MM cells. In addition, transcriptomic and cellular metabolic analysis demonstrated that CD38KO NK cells have unique metabolic reprogramming with higher mitochondrial respiratory capacity. Finally, we evaluated the impact of exposure to all-trans retinoic acid (ATRA) on wild-type NK and CD38KO NK cell function and highlighted potential benefits and drawbacks of combining ATRA with DARA in patients with MM. Taken together, these findings provide proof of concept that adoptive immunotherapy using ex vivo expanded CD38KO NK cells has the potential to boost DARA activity in MM.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.A.L. has consulting relationships with Caribou Biosciences and Courier Therapeutics, and equity/leadership/consulting relationships with Kiadis Pharma Netherlands B.V. The remaining authors declare no competing financial interests.
Figures


References
-
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324-339. - PubMed
-
- Laubach J, Garderet L, Mahindra A, et al. . Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016;30(5):1005-1017. - PubMed
-
- Kumar SK, Dimopoulos MA, Kastritis E, et al. . Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443-2448. - PubMed
-
- Dimopoulos MA, Oriol A, Nahi H, et al. ; POLLUX Investigators . Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

