Metabolic Syndrome and COVID 19: Endocrine-Immune-Vascular Interactions Shapes Clinical Course
- PMID: 32603424
- PMCID: PMC7337756
- DOI: 10.1210/endocr/bqaa112
Metabolic Syndrome and COVID 19: Endocrine-Immune-Vascular Interactions Shapes Clinical Course
Abstract
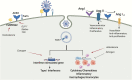
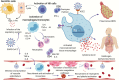
The ongoing coronavirus disease 2019 (COVID-19) pandemic is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Individuals with metabolic syndrome are at increased risk for poor disease outcomes and mortality from COVID-19. The pathophysiologic mechanisms for these observations have not been fully elucidated. A critical interaction between SARS-CoV-2 and the angiotensin-converting enzyme 2 (ACE2) facilitates viral entry into the host cell. ACE2 is expressed in pancreatic islets, vascular endothelium, and adipose tissue, and the SARS-CoV-2 -ACE2 interaction in these tissues, along with other factors, governs the spectrum and the severity of clinical manifestations among COVID-19 patients with metabolic syndrome. Moreover, the pro-inflammatory milieu observed in patients with metabolic syndrome may contribute toward COVID-19-mediated host immune dysregulation, including suboptimal immune responses, hyperinflammation, microvascular dysfunction, and thrombosis. This review describes the spectrum of clinical features, the likely pathophysiologic mechanisms, and potential implications for the management of metabolic syndrome in COVID-19 patients.
Keywords: COVID-19; coronavirus; diabetes; hypertension; metabolic syndrome; obesity.
Published by Oxford University Press on behalf of the Endocrine Society 2020.
Figures


Comment in
-
Metabolic Syndrome and COVID-19: Endocrine-Immune Vascular Interactions Shape the Clinical Course.Endocrinology. 2020 Dec 1;161(12):bqaa131. doi: 10.1210/endocr/bqaa131. Endocrinology. 2020. PMID: 33202024 Free PMC article. No abstract available.
References
-
- Grundy SM. Metabolic syndrome. In: Bonora E, DeFronzo RA, eds. Diabetes Complications, Comorbidities and Related Disorders. Cham: Springer International Publishing; 2020:71–107.
-
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-1776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

