Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for Treatment of Anemia in Chronic Kidney Disease: A Placebo-Controlled Study of Pharmacokinetic and Pharmacodynamic Profiles in Hemodialysis Patients
- PMID: 32603526
- PMCID: PMC7586807
- DOI: 10.1002/jcph.1648
Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for Treatment of Anemia in Chronic Kidney Disease: A Placebo-Controlled Study of Pharmacokinetic and Pharmacodynamic Profiles in Hemodialysis Patients
Abstract
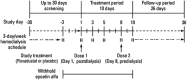
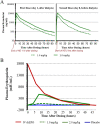
Roxadustat (FG-4592), an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that stimulates erythropoiesis, was evaluated in a phase 1b study in patients with end-stage renal disease with anemia on hemodialysis. Seventeen patients, on epoetin-alfa maintenance therapy with stable hemoglobin levels ≥10 g/dL, had epoetin-alfa discontinued on day 3 and were enrolled in this double-blind placebo-controlled study. Two cohorts were randomized 3:1 (roxadustat: placebo). Patients received single doses of roxadustat (1 or 2 mg/kg) or placebo 1 hour after hemodialysis on day 1 and 2 hours before dialysis on day 8. Maximum plasma concentration and area under the plasma concentration-time curve for patients receiving roxadustat were slightly more than dose proportional and elimination half-life ranged from 14.7 to 19.4 hours. Roxadustat was highly protein bound (99%) in plasma, and dialysis contributed a small fraction of the total clearance: only 4.56% and 3.04% of roxadustat recovered from the 1 and 2 mg/kg dose groups, respectively. Roxadustat induced transient elevations of endogenous erythropoietin that peaked between 7 and 14 hours after dosing and returned to baseline by 48 hours after dosing. Peak median endogenous erythropoietin levels were 96 mIU/mL and 268 mIU/mL for the 1- and 2-mg/kg doses, respectively, within physiologic range of endogenous erythropoietin responses to hypoxia at high altitude or after blood loss. No serious adverse events were reported, and there were no treatment- or dose-related trends in adverse event incidence.
Keywords: anemia; dialysis; erythropoietin; pharmacokinetics; roxadustat.
© 2020 FibroGen. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
FibroGen Inc. was the sponsor of this study, and J.C., and K.H.P.Y. are employees of FibroGen and hold stock and/or stock options in FibroGen. At the time the study was performed, R.P. was affiliated with St. John Hospital & Medical Center and with Wayne State University School of Medicine. He currently is an employee and holds stock in DaVita Healthcare Partners. All other authors declare no conflicts of interest.
Figures
References
-
- US Center of Disease Control . National Chronic Kidney Disease Fact Sheet. Center for Disease Control and Prevention Website. https://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf. Published 2017. Accessed February 21, 2020.
-
- Smith RE, Jr . The clinical and economic burden of anemia. Am J Manag Care. 2010;16(suppl.):S59‐S66. - PubMed
-
- Nakhoul G, Simon JF. Anemia of chronic kidney disease: treat it, but not too aggressively. Cleve Clin J Med. 2016;83(8):613‐624. - PubMed
-
- Horl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol. 2013:9(5):291‐301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

