Corticosteroid administration for viral pneumonia: COVID-19 and beyond
- PMID: 32603802
- PMCID: PMC7320691
- DOI: 10.1016/j.cmi.2020.06.020
Corticosteroid administration for viral pneumonia: COVID-19 and beyond
Abstract
Background: Corticosteroids are commonly used as adjuvant therapy for acute respiratory distress syndrome by many clinicians because of their perceived anti-inflammatory effects. However, for patients with severe viral pneumonia, the corticosteroid treatment is highly controversial.
Objectives: The purpose of this review is to systematically evaluate the effect and potential mechanism of corticosteroid administration in pandemic viral pneumonia.
Sources: We comprehensively searched all manuscripts on corticosteroid therapy for influenza, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and SARS coronavirus 2 (SARS-CoV-2) viral pneumonia from the PubMed, EMBASE, Web of Science and Cochrane Library databases.
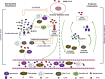
Content: We systematically summarized the effects of corticosteroid therapy for pandemic viral pneumonia and the potential mechanism of action for corticosteroids in coronavirus disease 2019 (COVID-19).
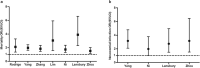
Implications: Observational studies showed that corticosteroid treatment was associated with increased mortality and nosocomial infections for influenza and delayed virus clearance for SARS-CoV and MERS-CoV. Limited data on corticosteroid therapy for COVID-19 were reported. Corticosteroids were used in about a fifth of patients (670/2995, 22.4%). Although clinical observational studies reported the improvement in symptoms and oxygenation for individuals with severe COVID-19 who received corticosteroid therapy, case fatality rate in the corticosteroid group was significantly higher than that in the non-corticosteroid group (69/443, 15.6% versus 56/1310, 4.3%). Compared individuals with non-severe disease, those with severe disease were more likely to receive corticosteroid therapy (201/382, 52.6% versus 201/1310, 15.3%). Although there is no evidence that corticosteroid therapy reduces mortality in people with COVID-19, some improvements in clinical symptoms and oxygenation were reported in some clinical observational studies. Excessive inflammatory response and lymphopenia might be critical factors associated with severity of and mortality from COVID-19. Sufficiently powered randomized controlled trials with rigorous inclusion/exclusion criteria and standardized dose and duration of corticosteroids are needed to verify the effectiveness and safety of corticosteroid therapy.
Keywords: COVID-19; Corticosteroid; Influenza; MERS; SARS.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis.Leukemia. 2020 Jun;34(6):1503-1511. doi: 10.1038/s41375-020-0848-3. Epub 2020 May 5. Leukemia. 2020. PMID: 32372026 Free PMC article.
-
Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis.CMAJ. 2020 Jul 6;192(27):E756-E767. doi: 10.1503/cmaj.200645. Epub 2020 May 14. CMAJ. 2020. PMID: 32409522 Free PMC article.
-
Corticosteroids as adjunctive therapy in the treatment of influenza.Cochrane Database Syst Rev. 2019 Feb 24;2(2):CD010406. doi: 10.1002/14651858.CD010406.pub3. Cochrane Database Syst Rev. 2019. PMID: 30798570 Free PMC article.
-
Corticosteroid use in viral pneumonia: experience so far and the dexamethasone breakthrough in coronavirus disease-2019.J Comp Eff Res. 2020 Dec;9(18):1247-1254. doi: 10.2217/cer-2020-0146. Epub 2020 Nov 27. J Comp Eff Res. 2020. PMID: 33245242 Free PMC article. Review.
-
COVID-19 and corticosteroids: a narrative review.Inflammopharmacology. 2022 Aug;30(4):1189-1205. doi: 10.1007/s10787-022-00987-z. Epub 2022 May 13. Inflammopharmacology. 2022. PMID: 35562628 Free PMC article. Review.
Cited by
-
An updated review on potential therapeutic drug candidates, vaccines and an insight on patents filed for COVID-19.Curr Res Pharmacol Drug Discov. 2021;2:100063. doi: 10.1016/j.crphar.2021.100063. Epub 2021 Oct 8. Curr Res Pharmacol Drug Discov. 2021. PMID: 34870158 Free PMC article. Review.
-
Influenza infection, SARS, MERS and COVID-19: Cytokine storm - The common denominator and the lessons to be learned.Clin Immunol. 2021 Feb;223:108652. doi: 10.1016/j.clim.2020.108652. Epub 2020 Dec 14. Clin Immunol. 2021. PMID: 33333256 Free PMC article. Review.
-
Patient and Clinical Factors at Admission Affect the Levels of Neutralizing Antibodies Six Months after Recovering from COVID-19.Viruses. 2022 Jan 2;14(1):80. doi: 10.3390/v14010080. Viruses. 2022. PMID: 35062284 Free PMC article.
-
Neutrophil extracellular traps (NETs): the role of inflammation and coagulation in COVID-19.Am J Transl Res. 2021 Aug 15;13(8):8575-8588. eCollection 2021. Am J Transl Res. 2021. PMID: 34539980 Free PMC article. Review.
-
Why Haven't We Found an Effective Treatment for COVID-19?Front Immunol. 2021 Mar 31;12:644850. doi: 10.3389/fimmu.2021.644850. eCollection 2021. Front Immunol. 2021. PMID: 33868280 Free PMC article. Review. No abstract available.
References
-
- WHO . 2020. Coronavirus disease (COVID-19) outbreak situation.http://covid19.who.int 30 April. Available from:
-
- Chan E.D., Chan M.M., Chan M.M., Marik P.E. Use of glucocorticoids in the critical care setting: science and clinical evidence. Pharmacol Ther. 2020;206:107428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous