Controversy of physiological vs. pharmacological effects of BMP signaling: Constitutive activation of BMP type IA receptor-dependent signaling in osteoblast lineage enhances bone formation and resorption, not affecting net bone mass
- PMID: 32603910
- PMCID: PMC7423725
- DOI: 10.1016/j.bone.2020.115513
Controversy of physiological vs. pharmacological effects of BMP signaling: Constitutive activation of BMP type IA receptor-dependent signaling in osteoblast lineage enhances bone formation and resorption, not affecting net bone mass
Abstract
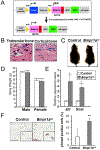
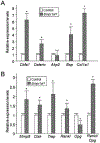
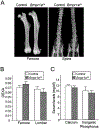
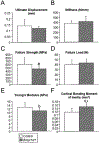
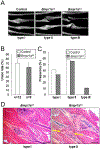
Bone morphogenetic proteins (BMPs) were first described over 50 years ago as potent inducers of ectopic bone formation when administrated subcutaneously. Preclinical studies have extensively examined the osteoinductive properties of BMPs in vitro and new bone formation in vivo. BMPs (BMP-2, BMP-7) have been used in orthopedics over 15 years. While osteogenic function of BMPs has been widely accepted, our previous studies demonstrated that loss-of-function of BMP receptor type IA (BMPR1A), a potent receptor for BMP-2, increased net bone mass by significantly inhibiting bone resorption in mice, indicating a positive role of BMP signaling in bone resorption. The physiological role of BMPs (i.e. osteogenic vs. osteoclastogenic) is still largely unknown. The purpose of this study was to investigate the physiological role of BMP signaling in endogenous long bones during adult stages. For this purpose, we conditionally and constitutively activated the Smad-dependent canonical BMP signaling thorough BMPR1A in osteoblast lineage cells using the mutant mice (Col1CreER™:caBmpr1a). Because trabecular bones were largely increased in the loss-of-function mouse study for BMPR1A, we hypothesized that the augmented BMP signaling would affect endogenous trabecular bones. In the mutant bones, the Smad phosphorylation was enhanced within physiological level three-fold while the resulting gross morphology, bodyweights, bone mass/shape/length, serum calcium/phosphorus levels, collagen cross-link patterns, and healing capability were all unchanged. Interestingly, we found; 1) increased expressions of both bone formation and resorption markers in femoral bones, 2) increased osteoblast and osteoclast numbers together with dynamic bone formation parameters by trabecular bone histomorphometry, 3) modest bone architectural phenotype with reduced bone quality (i.e. reduced trabecular bone connectivity, larger diametric size but reduced cortical bone thickness, and reduced bone mechanical strength), and 4) increased expression of SOST, a downstream target of the Smad-dependent BMPR1A signaling, in the mutant bones. This study is clinically insightful because gain-of-function of BMP signaling within a physiological window does not increase bone mass while it alters molecular and cellular aspects of osteoblast and osteoclast functions as predicted. These findings help explain the high-doses of BMPs (i.e. pharmacological level) in clinical settings required to substantially induce a bone formation, concurrent with potential unexpected side effects (i.e. bone resorption, inflammation) presumably due to a broader population of cell-types exposed to the high-dose BMPs rather than osteoblastic lineage cells.
Keywords: BMPR1A; Bone morphogenetic protein; Osteoblast lineage cells; Pharmacologic; Physiologic.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure All authors state that they have no conflicts of interest. Some of the animal experiments were performed at the National Institute of Environmental Health Sciences, NIH, while NK and YM were affiliated at the institution.
Figures


Similar articles
-
Increased BMP-Smad signaling does not affect net bone mass in long bones.Front Physiol. 2023 Mar 30;14:1145763. doi: 10.3389/fphys.2023.1145763. eCollection 2023. Front Physiol. 2023. PMID: 37064883 Free PMC article.
-
BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway.Development. 2008 Nov;135(22):3801-11. doi: 10.1242/dev.025825. Epub 2008 Oct 16. Development. 2008. PMID: 18927151 Free PMC article.
-
Loss of BMP signaling through BMPR1A in osteoblasts leads to greater collagen cross-link maturation and material-level mechanical properties in mouse femoral trabecular compartments.Bone. 2016 Jul;88:74-84. doi: 10.1016/j.bone.2016.04.022. Epub 2016 Apr 23. Bone. 2016. PMID: 27113526 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications.Front Immunol. 2022 Apr 26;13:869422. doi: 10.3389/fimmu.2022.869422. eCollection 2022. Front Immunol. 2022. PMID: 35558080 Free PMC article. Review.
Cited by
-
Increased BMP-Smad signaling does not affect net bone mass in long bones.Front Physiol. 2023 Mar 30;14:1145763. doi: 10.3389/fphys.2023.1145763. eCollection 2023. Front Physiol. 2023. PMID: 37064883 Free PMC article.
-
Gain-of-Function of FGFR3 Accelerates Bone Repair Following Ischemic Osteonecrosis in Juvenile Mice.Calcif Tissue Int. 2022 Dec;111(6):622-633. doi: 10.1007/s00223-022-01019-2. Epub 2022 Sep 7. Calcif Tissue Int. 2022. PMID: 36069912
-
Augmentation of BMP Signaling in Cranial Neural Crest Cells Leads to Premature Cranial Sutures Fusion through Endochondral Ossification in Mice.JBMR Plus. 2023 Feb 23;7(4):e10716. doi: 10.1002/jbm4.10716. eCollection 2023 Apr. JBMR Plus. 2023. PMID: 37065628 Free PMC article.
-
Targeting the BMP Pathway in Prostate Cancer Induced Bone Disease.Front Endocrinol (Lausanne). 2021 Dec 10;12:769316. doi: 10.3389/fendo.2021.769316. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34956082 Free PMC article.
-
Impact of PEG sensitization on the efficacy of PEG hydrogel-mediated tissue engineering.Nat Commun. 2024 Apr 18;15(1):3283. doi: 10.1038/s41467-024-46327-3. Nat Commun. 2024. PMID: 38637507 Free PMC article.
References
-
- Urist MR, Bone: formation by autoinduction. Science, 1965. 150(698): p. 893–9. - PubMed
-
- Wozney JM, et al., Novel regulators of bone formation: molecular clones and activities. Science, 1988. 242(4885): p. 1528–34. - PubMed
-
- Luyten FP, et al., Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem, 1989. 264(23): p. 13377–80. - PubMed
-
- Wozney JM, The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev, 1992. 32(2): p. 160–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

