Maternal Tryptophan Supplementation Protects Adult Rat Offspring against Hypertension Programmed by Maternal Chronic Kidney Disease: Implication of Tryptophan-Metabolizing Microbiome and Aryl Hydrocarbon Receptor
- PMID: 32604820
- PMCID: PMC7349830
- DOI: 10.3390/ijms21124552
Maternal Tryptophan Supplementation Protects Adult Rat Offspring against Hypertension Programmed by Maternal Chronic Kidney Disease: Implication of Tryptophan-Metabolizing Microbiome and Aryl Hydrocarbon Receptor
Abstract
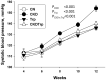
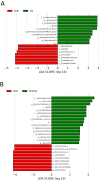
Hypertension and chronic kidney disease (CKD) can originate during early-life. Tryptophan metabolites generated by different pathways have both detrimental and beneficial effects. In CKD, uremic toxins from the tryptophan-generating metabolites are endogenous ligands of the aryl hydrocarbon receptor (AHR). The interplay between AHR, nitric oxide (NO), the renin-angiotensin system (RAS), and gut microbiota is involved in the development of hypertension. We examined whether tryptophan supplementation in pregnancy can prevent hypertension and kidney disease programmed by maternal CKD in adult offspring via the aforementioned mechanisms. Sprague-Dawley (SD) female rats received regular chow or chow supplemented with 0.5% adenine for 3 weeks to induce CKD before pregnancy. Pregnant controls or CKD rats received vehicle or tryptophan 200 mg/kg per day via oral gavage during pregnancy. Male offspring were divided into four groups (n = 8/group): control, CKD, tryptophan supplementation (Trp), and CKD plus tryptophan supplementation (CKDTrp). All rats were sacrificed at the age of 12 weeks. We found maternal CKD induced hypertension in adult offspring, which tryptophan supplementation prevented. Maternal CKD-induced hypertension is related to impaired NO bioavailability and non-classical RAS axis. Maternal CKD and tryptophan supplementation differentially shaped distinct gut microbiota profile in adult offspring. The protective effect of tryptophan supplementation against maternal CKD-induced programmed hypertension is relevant to alterations to several tryptophan-metabolizing microbes and AHR signaling pathway. Our findings support interplay among tryptophan-metabolizing microbiome, AHR, NO, and the RAS in hypertension of developmental origins. Furthermore, tryptophan supplementation in pregnancy could be a potential approach to prevent hypertension programmed by maternal CKD.
Keywords: aryl hydrocarbon receptor; chronic kidney disease; developmental origins of health and disease (DOHaD); gut microbiota; hypertension; nitric oxide; pregnancy; renin–angiotensin system; tryptophan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

