Targeting Cardiac Stem Cell Senescence to Treat Cardiac Aging and Disease
- PMID: 32604861
- PMCID: PMC7349658
- DOI: 10.3390/cells9061558
Targeting Cardiac Stem Cell Senescence to Treat Cardiac Aging and Disease
Abstract
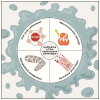
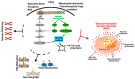
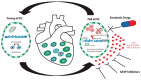
Adult stem/progenitor are a small population of cells that reside in tissue-specific niches and possess the potential to differentiate in all cell types of the organ in which they operate. Adult stem cells are implicated with the homeostasis, regeneration, and aging of all tissues. Tissue-specific adult stem cell senescence has emerged as an attractive theory for the decline in mammalian tissue and organ function during aging. Cardiac aging, in particular, manifests as functional tissue degeneration that leads to heart failure. Adult cardiac stem/progenitor cell (CSC) senescence has been accordingly associated with physiological and pathological processes encompassing both non-age and age-related decline in cardiac tissue repair and organ dysfunction and disease. Senescence is a highly active and dynamic cell process with a first classical hallmark represented by its replicative limit, which is the establishment of a stable growth arrest over time that is mainly secondary to DNA damage and reactive oxygen species (ROS) accumulation elicited by different intrinsic stimuli (like metabolism), as well as external stimuli and age. Replicative senescence is mainly executed by telomere shortening, the activation of the p53/p16INK4/Rb molecular pathways, and chromatin remodeling. In addition, senescent cells produce and secrete a complex mixture of molecules, commonly known as the senescence-associated secretory phenotype (SASP), that regulate most of their non-cell-autonomous effects. In this review, we discuss the molecular and cellular mechanisms regulating different characteristics of the senescence phenotype and their consequences for adult CSCs in particular. Because senescent cells contribute to the outcome of a variety of cardiac diseases, including age-related and unrelated cardiac diseases like diabetic cardiomyopathy and anthracycline cardiotoxicity, therapies that target senescent cell clearance are actively being explored. Moreover, the further understanding of the reversibility of the senescence phenotype will help to develop novel rational therapeutic strategies.
Keywords: SASP; adult stem cells; aging; epigenetics; metabolism; senescence; tissue homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Shay J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016;6:584–593. doi: 10.1158/2159-8290.CD-16-0062. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

