Mistletoe-Extract Drugs Stimulate Anti-Cancer Vγ9Vδ2 T Cells
- PMID: 32604868
- PMCID: PMC7349316
- DOI: 10.3390/cells9061560
Mistletoe-Extract Drugs Stimulate Anti-Cancer Vγ9Vδ2 T Cells
Abstract
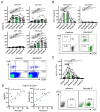
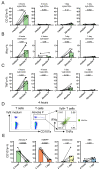
Human phosphoantigen-reactive Vγ9Vδ2 T cells possess several characteristics, including MHC-independent recognition of tumor cells and potent killing potential, that make them attractive candidates for cancer immunotherapeutic approaches. Injectable preparations from the hemi-parasite plant Viscum album L. (European mistletoe) are commonly prescribed as complementary cancer therapy in European countries such as Germany, but their mechanism of action remains poorly understood. Here, we investigated in-depth the in vitro response of human T cells towards mistletoe-extract drugs by analyzing their functional and T-cell-receptor (TCR) response using flow cytometry and high-throughput sequencing respectively. Non-fermented mistletoe-extract drugs (AbnobaViscum), but not their fermented counterparts (Iscador), induced specific expansion of Vγ9Vδ2 T cells among T cells. Furthermore, AbnobaViscum rapidly induced the release of cytotoxic granules and the production of the cytokines IFNγ and TNFα in Vγ9Vδ2 T cells. This stimulation of anti-cancer Vγ9Vδ2 T cells was mediated by the butyrophilin BTN3A, did not depend on the accumulation of endogenous phosphoantigens and involved the same Vγ9Vδ2 TCR repertoire as those of phosphoantigen-reactive Vγ9Vδ2 T cells. These insights highlight Vγ9Vδ2 T cells as a potential target for mistletoe-extract drugs and their role in cancer patients receiving these herbal drugs needs to be investigated.
Keywords: T cell receptor; TCR; Vgamma9Vdelta2; gammadelta; herbal drug; mistletoe.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

