Hyperthermia Disturbs and Delays Spontaneous Differentiation of Human Embryoid Bodies
- PMID: 32604871
- PMCID: PMC7345654
- DOI: 10.3390/biomedicines8060176
Hyperthermia Disturbs and Delays Spontaneous Differentiation of Human Embryoid Bodies
Abstract
Various types of stress stimuli have been shown to threaten the normal development of embryos during embryogenesis. Prolonged heat exposure is the most common stressor that poses a threat to embryo development. Despite the extensive investigation of heat stress control mechanisms in the cytosol, the endoplasmic reticulum (ER) heat stress response remains unclear. In this study, we used human embryonic stem cells (hESCs) to examine the effect of heat stress on early embryonic development, specifically alterations in the ER stress response. In a hyperthermic (42 °C) culture, ER stress response genes involved in hESC differentiation were induced within 1 h of exposure, which resulted in disturbed and delayed differentiation. In addition, hyperthermia increased the expression levels of activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) genes, which are associated with the protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway. Furthermore, we demonstrated that tauroursodeoxycholic acid, a chemical chaperone, mitigated the delayed differentiation under hyperthermia. Our study identified novel gene markers in response to hyperthermia-induced ER stress on hESCs, thereby providing further insight into the mechanisms that regulate human embryogenesis.
Keywords: embryogenesis; endoplasmic reticulum; human embryonic stem cells; hyperthermia; stress response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

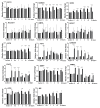

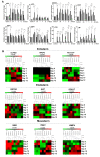
References
LinkOut - more resources
Full Text Sources
Research Materials

