Reciprocal Regulation between Primary Cilia and mTORC1
- PMID: 32604881
- PMCID: PMC7349257
- DOI: 10.3390/genes11060711
Reciprocal Regulation between Primary Cilia and mTORC1
Abstract
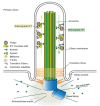
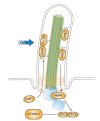
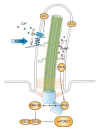
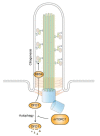
In quiescent cells, primary cilia function as a mechanosensor that converts mechanic signals into chemical activities. This unique organelle plays a critical role in restricting mechanistic target of rapamycin complex 1 (mTORC1) signaling, which is essential for quiescent cells to maintain their quiescence. Multiple mechanisms have been identified that mediate the inhibitory effect of primary cilia on mTORC1 signaling. These mechanisms depend on several tumor suppressor proteins localized within the ciliary compartment, including liver kinase B1 (LKB1), AMP-activated protein kinase (AMPK), polycystin-1, and polycystin-2. Conversely, changes in mTORC1 activity are able to affect ciliogenesis and stability indirectly through autophagy. In this review, we summarize recent advances in our understanding of the reciprocal regulation of mTORC1 and primary cilia.
Keywords: AMPK; LKB1; Tsc2; autophagy; ciliogenesis; mTOR; mTORC1; polycystin-1; primary cilia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

