Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation
- PMID: 32604886
- PMCID: PMC7355739
- DOI: 10.3390/biom10060964
Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation
Abstract
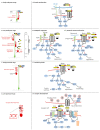
During embryonic development and adulthood, Reelin exerts several important functions in the brain including the regulation of neuronal migration, dendritic growth and branching, dendritic spine formation, synaptogenesis and synaptic plasticity. As a consequence, the Reelin signaling pathway has been associated with several human brain disorders such as lissencephaly, autism, schizophrenia, bipolar disorder, depression, mental retardation, Alzheimer's disease and epilepsy. Several elements of the signaling pathway are known. Core components, such as the Reelin receptors very low-density lipoprotein receptor (VLDLR) and Apolipoprotein E receptor 2 (ApoER2), Src family kinases Src and Fyn, and the intracellular adaptor Disabled-1 (Dab1), are common to most but not all Reelin functions. Other downstream effectors are, on the other hand, more specific to defined tasks. Reelin is a large extracellular protein, and some aspects of the signal are regulated by its processing into smaller fragments. Rather than being inhibitory, the processing at two major sites seems to be fulfilling important physiological functions. In this review, I describe the various cellular events regulated by Reelin and attempt to explain the current knowledge on the mechanisms of action. After discussing the shared and distinct elements of the Reelin signaling pathway involved in neuronal migration, dendritic growth, spine development and synaptic plasticity, I briefly outline the data revealing the importance of Reelin in human brain disorders.
Keywords: Reelin; cellular pathways; cerebral cortex; dendrites; embryonic development; migration; neurodevelopmental disorders; neuron; postnatal maturation; proteolytic processing; signal transduction; synapse.
Conflict of interest statement
The author declares no competing financial interests.
Figures

References
-
- De Rouvroit C.L., Goffinet A.M. Factors Influencing Mammalian Kidney Development: Implications for Health in Adult Life. Volume 150. Springer Science and Business Media; Berlin/Heidelberg, Germany: 1998. The Reeler Mouse as a Model of Brain Development; pp. 1–106. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

