Fast and Accurate Pneumocystis Pneumonia Diagnosis in Human Samples Using a Label-Free Plasmonic Biosensor
- PMID: 32604931
- PMCID: PMC7353103
- DOI: 10.3390/nano10061246
Fast and Accurate Pneumocystis Pneumonia Diagnosis in Human Samples Using a Label-Free Plasmonic Biosensor
Abstract
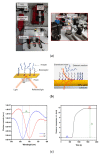
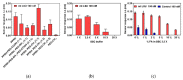
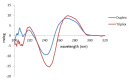
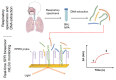
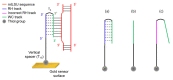
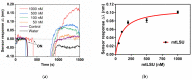
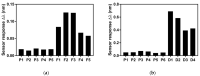
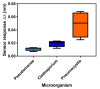
Pneumocystis jirovecii is a fungus responsible for human Pneumocystis pneumonia, one of the most severe infections encountered in immunodepressed individuals. The diagnosis of Pneumocystis pneumonia continues to be challenging due to the absence of specific symptoms in infected patients. Moreover, the standard diagnostic method employed for its diagnosis involves mainly PCR-based techniques, which besides being highly specific and sensitive, require specialized personnel and equipment and are time-consuming. Our aim is to demonstrate an optical biosensor methodology based on surface plasmon resonance to perform such diagnostics in an efficient and decentralized scheme. The biosensor methodology employs poly-purine reverse-Hoogsteen hairpin probes for the detection of the mitochondrial large subunit ribosomal RNA (mtLSU rRNA) gene, related to P. jirovecii detection. The biosensor device performs a real-time and label-free identification of the mtLSU rRNA gene with excellent selectivity and reproducibility, achieving limits of detection of around 2.11 nM. A preliminary evaluation of clinical samples showed rapid, label-free and specific identification of P. jirovecii in human lung fluids such as bronchoalveolar lavages or nasopharyngeal aspirates. These results offer a door for the future deployment of a sensitive diagnostic tool for fast, direct and selective detection of Pneumocystis pneumonia disease.
Keywords: DNA capture; Pneumocystis jirovecii; Surface Plasmon Resonance; clinical diagnosis; optical biosensor; triplex.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Wang H.W., Lin C.C., Kuo C.F., Liu C.P., Lee C.M. Mortality predictors of Pneumocystis jirovecii pneumonia in human immunodeficiency virus-infected patients at presentation: Experience in a tertiary care hospital of northern Taiwan. J. Microbiol. Immunol. Infect. 2011;44:274–281. doi: 10.1016/j.jmii.2010.08.006. - DOI - PubMed
Grants and funding
- SEV-2017-0706/Agencia Estatal de Investigación
- BES-2017-080527/Agencia Estatal de Investigación
- 2016-78515-R/Agencia Estatal de Investigación
- CYTED 212RT0450/Red Iberoamericana sobre Pneumocystosis in the framework of The Ibero-American Programme for Science, Technology and Development
- CTQ2017-84415-R/Ministerio de Ciencia e Innovación

