Validation of Breast Cancer Margins by Tissue Spray Mass Spectrometry
- PMID: 32604966
- PMCID: PMC7349349
- DOI: 10.3390/ijms21124568
Validation of Breast Cancer Margins by Tissue Spray Mass Spectrometry
Abstract
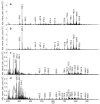
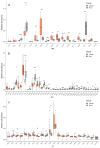
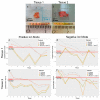
Current methods for the intraoperative determination of breast cancer margins commonly suffer from the insufficient accuracy, specificity and/or low speed of analysis, increasing the time and cost of operation as well the risk of cancer recurrence. The purpose of this study is to develop a method for the rapid and accurate determination of breast cancer margins using direct molecular profiling by mass spectrometry (MS). Direct molecular fingerprinting of tiny pieces of breast tissue (approximately 1 × 1 × 1 mm) is performed using a home-built tissue spray ionization source installed on a Maxis Impact quadrupole time-of-flight mass spectrometer (qTOF MS) (Bruker Daltonics, Hamburg, Germany). Statistical analysis of MS data from 50 samples of both normal and cancer tissue (from 25 patients) was performed using orthogonal projections onto latent structures discriminant analysis (OPLS-DA). Additionally, the results of OPLS classification of new 19 pieces of two tissue samples were compared with the results of histological analysis performed on the same tissues samples. The average time of analysis for one sample was about 5 min. Positive and negative ionization modes are used to provide complementary information and to find out the most informative method for a breast tissue classification. The analysis provides information on 11 lipid classes. OPLS-DA models are created for the classification of normal and cancer tissue based on the various datasets: All mass spectrometric peaks over 300 counts; peaks with a statistically significant difference of intensity determined by the Mann-Whitney U-test (p < 0.05); peaks identified as lipids; both identified and significantly different peaks. The highest values of Q2 have models built on all MS peaks and on significantly different peaks. While such models are useful for classification itself, they are of less value for building explanatory mechanisms of pathophysiology and providing a pathway analysis. Models based on identified peaks are preferable from this point of view. Results obtained by OPLS-DA classification of the tissue spray MS data of a new sample set (n = 19) revealed 100% sensitivity and specificity when compared to histological analysis, the "gold" standard for tissue classification. "All peaks" and "significantly different peaks" datasets in the positive ion mode were ideal for breast cancer tissue classification. Our results indicate the potential of tissue spray mass spectrometry for rapid, accurate and intraoperative diagnostics of breast cancer tissue as a means to reduce surgical intervention.
Keywords: breast cancer; direct mass spectrometry; discriminant model; lipidomics; molecular profiling; tissue spray.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Feature selection for OPLS discriminant analysis of cancer tissue lipidomics data.J Mass Spectrom. 2020 Jan;55(1):e4457. doi: 10.1002/jms.4457. Epub 2019 Dec 9. J Mass Spectrom. 2020. PMID: 31661719
-
Ambient ionization mass spectrometric analysis of human surgical specimens to distinguish renal cell carcinoma from healthy renal tissue.Anal Bioanal Chem. 2016 Aug;408(20):5407-14. doi: 10.1007/s00216-016-9627-4. Epub 2016 May 20. Anal Bioanal Chem. 2016. PMID: 27206411 Free PMC article.
-
Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery.Breast Cancer Res. 2017 May 23;19(1):59. doi: 10.1186/s13058-017-0845-2. Breast Cancer Res. 2017. PMID: 28535818 Free PMC article.
-
Surface analysis of lipids by mass spectrometry: more than just imaging.Prog Lipid Res. 2013 Oct;52(4):329-53. doi: 10.1016/j.plipres.2013.04.005. Epub 2013 Apr 24. Prog Lipid Res. 2013. PMID: 23623802 Review.
-
Advances in mass spectrometry for lipidomics.Annu Rev Anal Chem (Palo Alto Calif). 2010;3:433-65. doi: 10.1146/annurev.anchem.111808.073705. Annu Rev Anal Chem (Palo Alto Calif). 2010. PMID: 20636050 Review.
Cited by
-
Alterations in lipid profile upon uterine fibroids and its recurrence.Sci Rep. 2021 Jun 1;11(1):11447. doi: 10.1038/s41598-021-89859-0. Sci Rep. 2021. PMID: 34075062 Free PMC article. Clinical Trial.
-
Discovery of lipid profiles in plasma-derived extracellular vesicles as biomarkers for breast cancer diagnosis.Cancer Sci. 2023 Oct;114(10):4020-4031. doi: 10.1111/cas.15935. Epub 2023 Aug 22. Cancer Sci. 2023. PMID: 37608343 Free PMC article.
-
Analytical Platforms for the Determination of Phospholipid Turnover in Breast Cancer Tissue: Role of Phospholipase Activity in Breast Cancer Development.Metabolites. 2021 Jan 4;11(1):32. doi: 10.3390/metabo11010032. Metabolites. 2021. PMID: 33406793 Free PMC article. Review.
References
-
- Jeevan R., Cromwell D.A., Trivella M., Lawrence G., Kearins O., Pereira J., Sheppard C., Caddy C.M., Van Der Meulen J.H.P. Reoperation rates after breast conserving surgery for breast cancer among women in England: Retrospective study of hospital episode statistics. BMJ. 2012;345:e4505. doi: 10.1136/bmj.e4505. - DOI - PMC - PubMed
-
- Veronesi U., Cascinelli N., Mariani L., Greco M., Saccozzi R., Luini A., Aguilar M., Marubini E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

