Reliable Characterization of Organic & Pharmaceutical Compounds with High Resolution Monochromated EEL Spectroscopy
- PMID: 32605004
- PMCID: PMC7408036
- DOI: 10.3390/polym12071434
Reliable Characterization of Organic & Pharmaceutical Compounds with High Resolution Monochromated EEL Spectroscopy
Abstract
Organic and biological compounds (especially those related to the pharmaceutical industry) have always been of great interest for researchers due to their importance for the development of new drugs to diagnose, cure, treat or prevent disease. As many new API (active pharmaceutical ingredients) and their polymorphs are in nanocrystalline or in amorphous form blended with amorphous polymeric matrix (known as amorphous solid dispersion-ASD), their structural identification and characterization at nm scale with conventional X-Ray/Raman/IR techniques becomes difficult. During any API synthesis/production or in the formulated drug product, impurities must be identified and characterized. Electron energy loss spectroscopy (EELS) at high energy resolution by transmission electron microscope (TEM) is expected to be a promising technique to screen and identify the different (organic) compounds used in a typical pharmaceutical or biological system and to detect any impurities present, if any, during the synthesis or formulation process. In this work, we propose the use of monochromated TEM-EELS, to analyze selected peptides and organic compounds and their polymorphs. In order to validate EELS for fingerprinting (in low loss/optical region) and by further correlation with advanced DFT, simulations were utilized.
Keywords: EELS; TEM; amorphous; monochromator; organic; pharmaceutical; plasmon loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
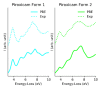
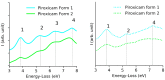
Similar articles
-
High-resolution monochromated electron energy-loss spectroscopy of organic photovoltaic materials.Ultramicroscopy. 2017 Sep;180:125-132. doi: 10.1016/j.ultramic.2017.03.004. Epub 2017 Mar 2. Ultramicroscopy. 2017. PMID: 28284703
-
Optimization of monochromated TEM for ultimate resolution imaging and ultrahigh resolution electron energy loss spectroscopy.Ultramicroscopy. 2018 Jan;184(Pt A):109-115. doi: 10.1016/j.ultramic.2017.08.016. Epub 2017 Sep 1. Ultramicroscopy. 2018. PMID: 28886488
-
Toward Developing a Predictive Approach To Assess Electron Beam Instability during Transmission Electron Microscopy of Drug Molecules.Mol Pharm. 2018 Nov 5;15(11):5114-5123. doi: 10.1021/acs.molpharmaceut.8b00693. Epub 2018 Sep 28. Mol Pharm. 2018. PMID: 30212216
-
Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions.Anal Bioanal Chem. 2017 Jul;409(18):4321-4333. doi: 10.1007/s00216-017-0292-z. Epub 2017 Mar 25. Anal Bioanal Chem. 2017. PMID: 28343348 Review.
-
Polymorphism and crystallization of active pharmaceutical ingredients (APIs).Curr Med Chem. 2009;16(7):884-905. doi: 10.2174/092986709787549299. Curr Med Chem. 2009. PMID: 19275600 Review.
Cited by
-
Accuracy and feasibility analysis of computational chemistry in drug spectral simulation-a case study of acetylsalicylic acid.BMC Chem. 2025 Jul 3;19(1):192. doi: 10.1186/s13065-025-01568-1. BMC Chem. 2025. PMID: 40611285 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

