Immunoadsorption and Plasma Exchange in Seropositive and Seronegative Immune-Mediated Neuropathies
- PMID: 32605107
- PMCID: PMC7409112
- DOI: 10.3390/jcm9072025
Immunoadsorption and Plasma Exchange in Seropositive and Seronegative Immune-Mediated Neuropathies
Abstract
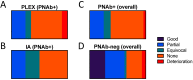
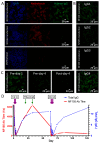
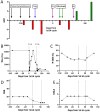
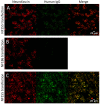
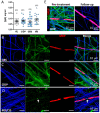
The inflammatory neuropathies are disabling conditions with diverse immunological mechanisms. In some, a pathogenic role for immunoglobulin G (IgG)-class autoantibodies is increasingly appreciated, and immunoadsorption (IA) may therefore be a useful therapeutic option. We reviewed the use of and response to IA or plasma exchange (PLEx) in a cohort of 41 patients with nodal/paranodal antibodies identified from a total of 573 individuals with suspected inflammatory neuropathies during the course of routine diagnostic testing (PNAb cohort). 20 patients had been treated with PLEx and 4 with IA. Following a global but subjective evaluation by their treating clinicians, none of these patients were judged to have had a good response to either of these treatment modalities. Sequential serology of one PNAb+ case suggests prolonged suppression of antibody levels with frequent apheresis cycles or adjuvant therapies, may be required for effective treatment. We further retrospectively evaluated the serological status of 40 patients with either Guillain-Barré syndrome (GBS) or chronic inflammatory demyelinating polyneuropathy (CIDP), and a control group of 20 patients with clinically-isolated syndrome/multiple sclerosis (CIS/MS), who had all been treated with IgG-depleting IA (IA cohort). 32 of these patients (8/20 with CIDP, 13/20 with GBS, 11/20 with MS) were judged responsive to apheresis despite none of the serum samples from this cohort testing positive for IgG antibodies against glycolipids or nodal/paranodal cell-adhesion molecules. Although negative on antigen specific assays, three patients' pre-treatment sera and eluates were reactive against different components of myelinating co-cultures. In summary, preliminary evidence suggests that GBS/CIDP patients without detectable IgG antibodies on routine diagnostic tests may nevertheless benefit from IA, and that an unbiased screening approach using myelinating co-cultures may assist in the detection of further autoantibodies which remain to be identified in such patients.
Keywords: Guillain-Barré syndrome; Inflammatory neuropathy; chronic inflammatory demyelinating polyneuropathy; immunoadsorption; multiple sclerosis; paranodal antibodies; plasma exchange; plasmapheresis.
Conflict of interest statement
S.R. runs a not-for-profit diagnostic testing service for nodal/paranodal antibodies. He has received a speaker’s honorarium and travel expenses from Fresenius Medical Care. A.D. is named inventor on a patent for immune cell therapy in nerve injury and has received travel grants from IASP and Biolegend. M.S. has received consulting and/or speaker honoraria from Bayer, Biogen, Merck, Roche, and Sanofi Genzyme. She has received research funding from the Hertha-Nathorff-Program. J.D. reports research funds and speaker’s honoraria from Fresenius Medical Care GmbH and Fresenius Medical Care Deutschland GmbH. The Globaffin IA column used to treat the NF155 PNAb+ patient was provided free of charge by Fresenius on a trial basis. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

