Nicotinamide-Rich Diet in DBA/2J Mice Preserves Retinal Ganglion Cell Metabolic Function as Assessed by PERG Adaptation to Flicker
- PMID: 32605122
- PMCID: PMC7401244
- DOI: 10.3390/nu12071910
Nicotinamide-Rich Diet in DBA/2J Mice Preserves Retinal Ganglion Cell Metabolic Function as Assessed by PERG Adaptation to Flicker
Abstract
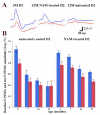
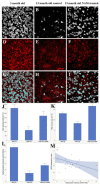
Flickering light increases metabolic demand in the inner retina. Flicker may exacerbate defective mitochondrial function in glaucoma, which will be reflected in the pattern electroretinogram (PERG), a sensitive test of retinal ganglion cell (RGC) function. We tested whether flicker altered the PERG of DBA/2J (D2) glaucomatous mice and whether vitamin B3-rich diet contributed to the flicker effect. D2 mice fed with either standard chow (control, n = 10) or chow/water enriched with nicotinamide (NAM, 2000 mg/kg per day) (treated, n = 10) were monitored from 3 to 12 months. The PERG was recorded with superimposed flicker (F-PERG) at either 101 Hz (baseline) or 11 Hz (test), and baseline-test amplitude difference (adaptation) evaluated. At endpoint, flat-mounted retinas were immunostained (RBPMS and mito-tracker). F-PERG adaptation was 41% in 3-month-old D2 and decreased with age more in control D2 than in NAM-fed D2 (GEE, p < 0.01). At the endpoint, F-PERG adaptation was 0% in control D2 and 17.5% in NAM-fed D2, together with higher RGC density (2.4×), larger RGC soma size (2×), and greater intensity of mitochondrial staining (3.75×). F-PERG adaptation may provide a non-invasive tool to assess RGC autoregulation in response to increased metabolic demand and test the effect of dietary/pharmacological treatments on optic nerve disorders.
Keywords: adaptation; mitochondria; nicotinamide; pattern electroretinogram; retinal ganglion cell.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

