Brachytherapy in a Single Dose of 10Gy as an "in situ" Vaccination
- PMID: 32605154
- PMCID: PMC7369911
- DOI: 10.3390/ijms21134585
Brachytherapy in a Single Dose of 10Gy as an "in situ" Vaccination
Abstract
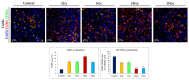
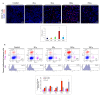
Radiotherapy (RT) is one of the major methods of cancer treatment. RT destroys cancer cells, but also affects the tumor microenvironment (TME). The delicate balance between immunomodulation processes in TME is dependent, among other things, on a specific radiation dose. Despite many studies, the optimal dose has not been clearly determined. Here, we demonstrate that brachytherapy (contact radiotherapy) inhibits melanoma tumor growth in a dose-dependent manner. Doses of 10Gy and 15Gy cause the most effective tumor growth inhibition compared to the control group. Brachytherapy, at a single dose of ≥ 5Gy, resulted in reduced tumor blood vessel density. Only a dose of 10Gy had the greatest impact on changes in the levels of tumor-infiltrating immune cells. It most effectively reduced the accumulation of protumorogenic M2 tumor-associated macrophages and increased the infiltration of cytotoxic CD8+ T lymphocytes. To summarize, more knowledge about the effects of irradiation doses in anticancer therapy is needed. It may help in the optimization of RT treatment. Our results indicate that a single dose of 10Gy leads to the development of a robust immune response. It seems that it is able to convert a tumor microenvironment into an "in situ" vaccine and lead to a significant inhibition of tumor growth.
Keywords: brachytherapy; dose; tumor microenvironment; tumor vasculature; “in situ” vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

