Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer
- PMID: 32605193
- PMCID: PMC7408037
- DOI: 10.3390/cells9071573
Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer
Abstract
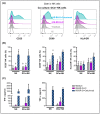
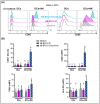
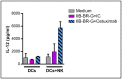
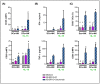
Triple Negative Breast Cancer (TNBC) treatment is still challenging, and immunotherapy is a potential approach in this tumor subtype. Cetuximab is an IgG1 monoclonal antibody (mAb) directed against Epidermic Growth Factor Receptor (EGFR), a protein overexpressed in a subgroup of TNBC patients and associated with poor prognosis. Previously, we demonstrated in vitro that Cetuximab triggers Ab-dependent cell cytotoxicity against TNBC cells. In this study, using co-cultures including TNBC cells, and NK and Dendritic Cells (DCs) from healthy donors, we studied the effect of Cetuximab-activated NK cells on DC function. Given that we already demonstrated that TNBC has an immunosuppressive effect on NK cells, we also tested Cetuximab combination with IL-15. We determined that Cetuximab opsonization of TNBC cells increased IFN-γ and TNF-α production by NK cells co-cultured with DCs. Moreover, we showed that NK cells activated by TNBC cells opsonized with Cetuximab promoted tumor material uptake and maturation of DCs, as well as their ability to produce IL-12. Furthermore, the stimulation with IL-15 increased the activation of NK cells and the maturation of DCs. These results suggest that IL-15 may enhance the efficacy of Cetuximab in the treatment of TNBC by promoting activation of both NK cells and DCs.
Keywords: ADCC; Cetuximab; NK cells; dendritic cells; triple negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Liedtke C., Mazouni C., Hess K.R., André F., Tordai A., Mejia J.A., Symmans W.F., Gonzalez-Angulo A.M., Hennessy B., Green M., et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. - DOI - PubMed
-
- Loi S., Drubay D., Adams S., Pruneri G., Francis P.A., Lacroix-Triki M., Joensuu H., Dieci M.V., Badve S., Demaria S., et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 2019;37:559–569. doi: 10.1200/JCO.18.01010. - DOI - PMC - PubMed
-
- Denkert C., von Minckwitz G., Brase J.C., Sinn B.V., Gade S., Kronenwett R., Pfitzner B.M., Salat C., Loi S., Schmitt W.D., et al. Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy With or Without Carboplatin in Human Epidermal Growth Factor Receptor 2–Positive and Triple-Negative Primary Breast Cancers. J. Clin. Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. - DOI - PubMed
-
- Kim I., Sanchez K., McArthur H.L., Page D. Immunotherapy in Triple-Negative Breast Cancer: Present and Future. Curr. Breast Cancer Rep. 2019;11:259–271. doi: 10.1007/s12609-019-00345-z. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

