NeuroHeal Reduces Muscle Atrophy and Modulates Associated Autophagy
- PMID: 32605216
- PMCID: PMC7408527
- DOI: 10.3390/cells9071575
NeuroHeal Reduces Muscle Atrophy and Modulates Associated Autophagy
Abstract
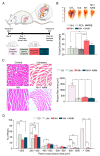
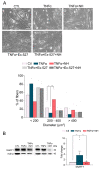
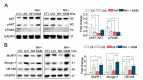
Muscle wasting is an unmet medical need which leads to a reduction of myofiber diameter and a negative impact on the functional performance of daily activities. We previously found that a new neuroprotective drug called NeuroHeal reduced muscle atrophy produced by transient denervation. Aiming to decipher whether NeuroHeal has a direct role in muscle biology, we used herein different models of muscle atrophy: one caused by chronic denervation, another caused by hindlimb immobilization, and lastly, an in vitro model of myotube atrophy with Tumor Necrosis Factor-α (TNFα). In all these models, we observed that NeuroHeal reduced muscle atrophy and that SIRT1 activation seems to be required for that. The treatment downregulated some critical markers of protein degradation: Muscle Ring Finger 1 (MuRF1), K48 poly-Ub chains, and p62/SQSTM1. Moreover, it seems to restore the autophagy flux associated with denervation. Hence, we envisage a prospective use of NeuroHeal at clinics for different myopathies.
Keywords: NeuroHeal; autophagy; proteasome; sirtuin 1; skeletal muscle atrophy.
Conflict of interest statement
The authors declare no conflict of interest. NeuroHeal use for muscle disorders is under patent review.
Figures


Similar articles
-
NeuroHeal Improves Muscle Regeneration after Injury.Cells. 2020 Dec 24;10(1):22. doi: 10.3390/cells10010022. Cells. 2020. PMID: 33374379 Free PMC article.
-
Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism.J Cachexia Sarcopenia Muscle. 2019 Apr;10(2):429-444. doi: 10.1002/jcsm.12393. Epub 2019 Feb 21. J Cachexia Sarcopenia Muscle. 2019. PMID: 30793539 Free PMC article.
-
Novel neuroprotective therapy with NeuroHeal by autophagy induction for damaged neonatal motoneurons.Theranostics. 2020 Apr 6;10(11):5154-5168. doi: 10.7150/thno.43765. eCollection 2020. Theranostics. 2020. PMID: 32308774 Free PMC article.
-
Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease.Int J Biochem Cell Biol. 2013 Oct;45(10):2245-56. doi: 10.1016/j.biocel.2013.06.015. Epub 2013 Jul 1. Int J Biochem Cell Biol. 2013. PMID: 23827718 Review.
-
The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy.Biochim Biophys Acta. 2008 Dec;1782(12):730-43. doi: 10.1016/j.bbadis.2008.10.011. Epub 2008 Oct 29. Biochim Biophys Acta. 2008. PMID: 18992328 Review.
Cited by
-
Introduction to the Special Issue "Skeletal Muscle Atrophy: Mechanisms at a Cellular Level".Cells. 2023 Feb 3;12(3):502. doi: 10.3390/cells12030502. Cells. 2023. PMID: 36766844 Free PMC article.
-
NeuroHeal Improves Muscle Regeneration after Injury.Cells. 2020 Dec 24;10(1):22. doi: 10.3390/cells10010022. Cells. 2020. PMID: 33374379 Free PMC article.
References
-
- Cohen I.R., Lambris J.D. Advances in Experimental Medicine and Biology. Springer Singapore. 2018;1088:1434–1436.
-
- Romeo-Guitart D., Forés J., Herrando-Grabulosa M., Valls R., Leiva-Rodríguez T., Galea E., González-Pérez F., Navarro X., Petegnief V., Bosch A., et al. Neuroprotective Drug for Nerve Trauma Revealed Using Artificial Intelligence. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-19767-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

