Efficacy of Panitumumab and Cetuximab in Patients with Colorectal Cancer Previously Treated with Bevacizumab; a Combined Analysis of Individual Patient Data from ASPECCT and WJOG6510G
- PMID: 32605298
- PMCID: PMC7407286
- DOI: 10.3390/cancers12071715
Efficacy of Panitumumab and Cetuximab in Patients with Colorectal Cancer Previously Treated with Bevacizumab; a Combined Analysis of Individual Patient Data from ASPECCT and WJOG6510G
Abstract
Background: Phase-III ASPECCT and randomised phase-II WJOG6510G trials demonstrated the noninferiority of panitumumab, when compared with cetuximab, for overall survival in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer.
Methods: The subgroup that received bevacizumab either prior to panitumumab or cetuximab monotherapy (ASPECCT) or in combination with irinotecan (WJOG6510G) was included. Multivariate Cox models were created, including the treatment arms as covariates together with patient, disease and treatment characteristics.
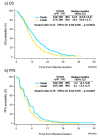
Results: We included 185 and 189 patients in the panitumumab and cetuximab arms, respectively. The median overall survival was 12.8 and 10.1 months [p = 0.0031; log-rank test, stratified by trial; hazard ratio (HR), 0.72; 95% confidence interval (CI), 0.58-0.90], and the median progression-free survival was 4.7 and 4.1 months, in the panitumumab and cetuximab arms, respectively (p = 0.0207; HR, 0.79; 95% CI, 0.64-0.97). The treatment regimen was an independent prognostic factor of overall survival (adjusted HR, 0.69; 95% CI, 0.54-0.87; p = 0.0013).
Conclusions: Panitumumab significantly prolonged the overall survival and progression-free survival, when compared with cetuximab in the cohort that previously received bevacizumab in the included studies. Clinical Trial Registration: ASPECCT trial registered with ClinicalTrials.gov (NCT01001377) and WJOG6510G trial registered with UMIN-CTR (UMIN000006643).
Keywords: anti-EGFR therapy; cetuximab; colorectal cancer; panitumumab.
Conflict of interest statement
HT reports receiving speakers bureau honoraria from Chugai, Takeda and Merck Serono. TY reports receiving grants from Chugai, Takeda and Merck Serono and speakers bureau honoraria from Chugai and Takeda. KY reports receiving speakers bureau honoraria from Chugai, Takeda and Merck Serono. KM reports grants from Merck Serono, personal fees from Chugai, and personal fees from Takeda. PR reports grants from Amgen. MP reports receiving speakers bureau honoraria from, and is a consultant/advisory board member for, Amgen. TP is a consultant/advisory board member for Amgen, Merck Serono and Takeda. The rest of the authors declare no conflict of interest.
Figures
References
-
- Karapetis C.S., Khambata-Ford S., Jonker D.J., O’Callaghan C.J., Tu D., Tebbutt N.C., Simes R.J., Chalchal H., Shapiro J.D., Robitaille S.M., et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. - DOI - PubMed
-
- Amado R.G., Wolf M., Peeters M., Cutsem E.V., Siena S., Freeman D.J., Juan T., Sikorski R., Suggs S., Patterson S.D., et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. - DOI - PubMed
-
- Price T.J., Peeters M., Kim T.W., Li J., Cascinu S., Ruff P., Suresh A.S., Thomas A., Tjulandin S., Zhang K., et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): A randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569–579. doi: 10.1016/S1470-2045(14)70118-4. - DOI - PubMed
-
- Price T., Kim T.W., Li J., Cascinu S., Ruff P., Suresh A.S., Thomas A., Tjulandin S., Guan X., Peeter M. Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur. J. Cancer. 2016;68:51–59. doi: 10.1016/j.ejca.2016.08.010. - DOI - PubMed
-
- Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C., et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

