Electrochemical Evaluation of a Multi-Site Clinical Depth Recording Electrode for Monitoring Cerebral Tissue Oxygen
- PMID: 32605324
- PMCID: PMC7407998
- DOI: 10.3390/mi11070632
Electrochemical Evaluation of a Multi-Site Clinical Depth Recording Electrode for Monitoring Cerebral Tissue Oxygen
Abstract
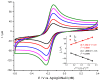
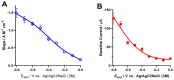
The intracranial measurement of local cerebral tissue oxygen levels-PbtO2-has become a useful tool for the critical care unit to investigate severe trauma and ischemia injury in patients. Our preliminary work in animal models supports the hypothesis that multi-site depth electrode recording of PbtO2 may give surgeons and critical care providers needed information about brain viability and the capacity for better recovery. Here, we present a surface morphology characterization and an electrochemical evaluation of the analytical properties toward oxygen detection of an FDA-approved, commercially available, clinical grade depth recording electrode comprising 12 Pt recording contacts. We found that the surface of the recording sites is composed of a thin film of smooth Pt and that the electrochemical behavior evaluated by cyclic voltammetry in acidic and neutral electrolyte is typical of polycrystalline Pt surface. The smoothness of the Pt surface was further corroborated by determination of the electrochemical active surface, confirming a roughness factor of 0.9. At an optimal working potential of -0.6 V vs. Ag/AgCl, the sensor displayed suitable values of sensitivity and limit of detection for in vivo PbtO2 measurements. Based on the reported catalytical properties of Pt toward the electroreduction reaction of O2, we propose that these probes could be repurposed for multisite monitoring of PbtO2 in vivo in the human brain.
Keywords: brain tissue oxygen; in vivo monitoring; multi-site clinical depth electrode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Ceramic-Based Multisite Platinum Microelectrode Arrays: Morphological Characteristics and Electrochemical Performance for Extracellular Oxygen Measurements in Brain Tissue.Anal Chem. 2017 Feb 7;89(3):1674-1683. doi: 10.1021/acs.analchem.6b03772. Epub 2017 Jan 25. Anal Chem. 2017. PMID: 28208270
-
Brain tissue oxygen tension and its response to physiological manipulations: influence of distance from injury site in a swine model of traumatic brain injury.J Neurosurg. 2016 Nov;125(5):1217-1228. doi: 10.3171/2015.7.JNS15809. Epub 2016 Feb 5. J Neurosurg. 2016. PMID: 26848909
-
A novel non-enzyme hydrogen peroxide sensor based on catalytic reduction property of silver nanowires.Talanta. 2015 Jul 1;139:56-61. doi: 10.1016/j.talanta.2015.02.037. Epub 2015 Feb 26. Talanta. 2015. PMID: 25882408
-
Comparison of jugular venous oxygen saturation and brain tissue Po2 as monitors of cerebral ischemia after head injury.Crit Care Med. 1999 Nov;27(11):2337-45. doi: 10.1097/00003246-199911000-00003. Crit Care Med. 1999. PMID: 10579245
-
Brain Tissue Oxygen Monitoring and the Intersection of Brain and Lung: A Comprehensive Review.Respir Care. 2016 Sep;61(9):1232-44. doi: 10.4187/respcare.04962. Epub 2016 Jul 19. Respir Care. 2016. PMID: 27435860 Review.
Cited by
-
Applications of flexible electronics related to cardiocerebral vascular system.Mater Today Bio. 2023 Aug 30;23:100787. doi: 10.1016/j.mtbio.2023.100787. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37766895 Free PMC article. Review.
-
Editorial on the Special Issue on Microelectrode Arrays and Application to Medical Devices.Micromachines (Basel). 2020 Aug 14;11(8):776. doi: 10.3390/mi11080776. Micromachines (Basel). 2020. PMID: 32823968 Free PMC article.
References
-
- Lang E.W., Jaeger M. Systematic and comprehensive literature review of publications on direct cerebral oxygenation monitoring. Open Crit. Care Med. J. 2013;6:1–24. doi: 10.2174/1874828701306010001. - DOI
-
- Bohman L.E., Pisapia J.M., Sanborn M.R., Frangos S., Lin E., Kumar M., Park S., Kofke W.A., Stiefel M.F., Leroux P.D., et al. Response of brain oxygen to therapy correlates with long-term outcome after subarachnoid hemorrhage. Neurocrit. Care. 2013;19:320–328. doi: 10.1007/s12028-013-9890-6. - DOI - PubMed
-
- Stiefel M.F., Spiotta A., Gracias V.H., Garuffe A.M., Guillamondegui O., Maloney-Wilensky E., Bloom S., Grady M.S., LeRoux P.D. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J. Neurosurg. 2005;103:805–811. doi: 10.3171/jns.2005.103.5.0805. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

