Cancer-Associated Point Mutations in the DLC1 Tumor Suppressor and Other Rho-GAPs Occur Frequently and Are Associated with Decreased Function
- PMID: 32606003
- PMCID: PMC8412439
- DOI: 10.1158/0008-5472.CAN-19-3984
Cancer-Associated Point Mutations in the DLC1 Tumor Suppressor and Other Rho-GAPs Occur Frequently and Are Associated with Decreased Function
Abstract
In advanced cancer, the RHOA GTPase is often active together with reduced expression of genes encoding Rho-specific GTPase-accelerating proteins (Rho-GAP), which negatively regulate RHOA and related GTPases. Here we used the The Cancer Genome Atlas dataset to examine 12 tumor types (including colon, breast, prostate, pancreas, lung adenocarcinoma, and squamous cell carcinoma) for the frequency of codon mutations of 10 Rho-GAP and experimentally tested biochemical and biological consequences for cancer-associated mutants that arose in the DLC1 tumor suppressor gene. DLC1 was the Rho-GAP gene mutated most frequently, with 5%-8% of tumors in five of the tumor types evaluated having DLC1 missense mutations. Furthermore, 20%-26% of the tumors in four of these five tumor types harbored missense mutations in at least one of the 10 Rho-GAPs. Experimental analysis of the DLC1 mutants indicated 7 of 9 mutants whose lesions were located in the Rho-GAP domain were deficient for Rho-GAP activity and for suppressing cell migration and anchorage-independent growth. Analysis of a DLC1 linker region mutant and a START domain mutant showed each was deficient for suppressing migration and growth in agar, but their Rho-GAP activity was similar to that of wild-type DLC1. Compared with the wild-type, the linker region mutant bound 14-3-3 proteins less efficiently, while the START domain mutant displayed reduced binding to Caveolin-1. Thus, mutation of Rho-GAP genes occurs frequently in some cancer types and the majority of cancer-associated DLC1 mutants evaluated were deficient biologically, with various mechanisms contributing to their reduced activity. SIGNIFICANCE: These findings indicate that point mutation of Rho-GAP genes is unexpectedly frequent in several cancer types, with DLC1 mutants exhibiting reduced function by various mechanisms.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures

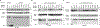


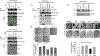
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

