Secondary Metabolism in the Gill Microbiota of Shipworms (Teredinidae) as Revealed by Comparison of Metagenomes and Nearly Complete Symbiont Genomes
- PMID: 32606027
- PMCID: PMC7329324
- DOI: 10.1128/mSystems.00261-20
Secondary Metabolism in the Gill Microbiota of Shipworms (Teredinidae) as Revealed by Comparison of Metagenomes and Nearly Complete Symbiont Genomes
Erratum in
-
Correction for Altamia et al., "Secondary Metabolism in the Gill Microbiota of Shipworms (Teredinidae) as Revealed by Comparison of Metagenomes and Nearly Complete Symbiont Genomes".mSystems. 2021 Apr 13;6(2):e00288-21. doi: 10.1128/mSystems.00288-21. mSystems. 2021. PMID: 33850046 Free PMC article. No abstract available.
Abstract
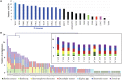
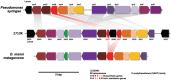
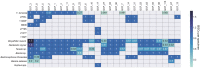
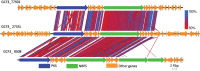
Shipworms play critical roles in recycling wood in the sea. Symbiotic bacteria supply enzymes that the organisms need for nutrition and wood degradation. Some of these bacteria have been grown in pure culture and have the capacity to make many secondary metabolites. However, little is known about whether such secondary metabolite pathways are represented in the symbiont communities within their hosts. In addition, little has been reported about the patterns of host-symbiont co-occurrence. Here, we collected shipworms from the United States, the Philippines, and Brazil and cultivated symbiotic bacteria from their gills. We analyzed sequences from 22 shipworm gill metagenomes from seven shipworm species and from 23 cultivated symbiont isolates. Using (meta)genome sequencing, we demonstrate that the cultivated isolates represent all the major bacterial symbiont species and strains in shipworm gills. We show that the bacterial symbionts are distributed among shipworm hosts in consistent, predictable patterns. The symbiotic bacteria harbor many gene cluster families (GCFs) for biosynthesis of bioactive secondary metabolites, only <5% of which match previously described biosynthetic pathways. Because we were able to cultivate the symbionts and to sequence their genomes, we can definitively enumerate the biosynthetic pathways in these symbiont communities, showing that ∼150 of ∼200 total biosynthetic gene clusters (BGCs) present in the animal gill metagenomes are represented in our culture collection. Shipworm symbionts occur in suites that differ predictably across a wide taxonomic and geographic range of host species and collectively constitute an immense resource for the discovery of new biosynthetic pathways corresponding to bioactive secondary metabolites.IMPORTANCE We define a system in which the major symbionts that are important to host biology and to the production of secondary metabolites can be cultivated. We show that symbiotic bacteria that are critical to host nutrition and lifestyle also have an immense capacity to produce a multitude of diverse and likely novel bioactive secondary metabolites that could lead to the discovery of drugs and that these pathways are found within shipworm gills. We propose that, by shaping associated microbial communities within the host, the compounds support the ability of shipworms to degrade wood in marine environments. Because these symbionts can be cultivated and genetically manipulated, they provide a powerful model for understanding how secondary metabolism impacts microbial symbiosis.
Keywords: biosynthesis; metagenomics.
Copyright © 2020 Altamia et al.
Figures





References
-
- Distel DL, Amin M, Burgoyne A, Linton E, Mamangkey G, Morrill W, Nove J, Wood N, Yang J. 2011. Molecular phylogeny of Pholadoidea Lamarck, 1809 supports a single origin for xylotrophy (wood feeding) and xylotrophic bacterial endosymbiosis in Bivalvia. Mol Phylogenet Evol 61:245–254. doi: 10.1016/j.ympev.2011.05.019. - DOI - PubMed
-
- Turner RD. 1966. A survey and illustrated catalogue of the Teredinidae (Mollusca: Bivalvia). Harvard University Press, Cambridge, MA.
-
- Luyten YA, Thompson JR, Morrill W, Polz MF, Distel DL. 2006. Extensive variation in intracellular symbiont community composition among members of a single population of the wood-boring bivalve Lyrodus pedicellatus (Bivalvia: Teredinidae). Appl Environ Microbiol 72:412–417. doi: 10.1128/AEM.72.1.412-417.2006. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
