Neuroendovascular clinical trials disruptions due to COVID-19. Potential future challenges and opportunities
- PMID: 32606103
- PMCID: PMC7371488
- DOI: 10.1136/neurintsurg-2020-016502
Neuroendovascular clinical trials disruptions due to COVID-19. Potential future challenges and opportunities
Abstract
To assess the impact of COVID-19 on neurovascular research and deal with the challenges imposed by the pandemic.
Methods: A survey-based study focused on randomized controlled trials (RCTs) and single-arm studies for acute ischemic stroke and cerebral aneurysms was developed by a group of senior neurointerventionalists and sent to sites identified through the clinical trials website (https://clinicaltrials.gov/), study sponsors, and physician investigators.
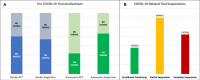
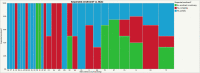
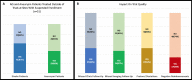
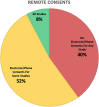
Results: The survey was sent to 101 institutions, with 65 responding (64%). Stroke RCTs were being conducted at 40 (62%) sites, aneurysm RCTs at 22 (34%) sites, stroke single-arm studies at 37 (57%) sites, and aneurysm single-arm studies at 43 (66%) sites. Following COVID-19, enrollment was suspended at 51 (78%) sites-completely at 21 (32%) and partially at 30 (46%) sites. Missed trial-related clinics and imaging follow-ups and protocol deviations were reported by 27 (42%), 24 (37%), and 27 (42%) sites, respectively. Negative reimbursements were reported at 17 (26%) sites. The majority of sites, 49 (75%), had put new trials on hold. Of the coordinators, 41 (63%) worked from home and 20 (31%) reported a personal financial impact. Remote consent was possible for some studies at 34 (52%) sites and for all studies at 5 (8%) sites. At sites with suspended trials (n=51), endovascular treatment without enrollment occurred at 31 (61%) sites for stroke and 23 (45%) sites for aneurysms. A total of 277 patients with acute ischemic stroke and 184 with cerebral aneurysms were treated without consideration for trial enrollment.
Conclusion: Widespread disruption of neuroendovascular trials occurred because of COVID-19. As sites resume clinical research, steps to mitigate similar challenges in the future should be considered.
Keywords: aneurysm; standards; stroke.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
