Biological Properties of the Aggregated Form of Chitosan Magnetic Nanoparticle
- PMID: 32606141
- PMCID: PMC7439901
- DOI: 10.21873/invivo.11966
Biological Properties of the Aggregated Form of Chitosan Magnetic Nanoparticle
Abstract
Background/aim: Chitosan-coated iron oxide nanoparticles (Chi-NP) have gained attention because of their biocompatibility, biodegradability, low toxicity and targetability under magnetic field. In this study, we investigated various biological properties of Chi-NP.
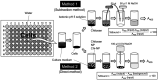
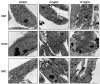
Materials and methods: Chi-NP was prepared by mixing magnetic NP with chitosan FL-80. Particle size was determined by scanning and transmission electron microscopes, cell viability by MTT assay, cell cycle distribution by cell sorter, synergism with anticancer drugs by combination index, PGE2 production in human gingival fibroblast was assayed by ELISA.
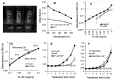
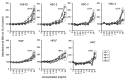
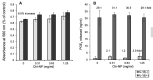
Results: The synthetic process of Chi-NP from FL-80 and magnetic NP increased the affinity to cells, up to the level attained by nanofibers. Upon contact with the culture medium, Chi-NP instantly formed aggregates and interfered with intracellular uptake. Aggregated Chi-NP did not show cytotoxicity, synergism with anticancer drugs, induce apoptosis (accumulation of subG1 cell population), protect the cells from X-ray-induced damage, nor affected both basal and IL-1β-induced PGE2 production.
Conclusion: Chi-NP is biologically inert and shows high affinity to cells, further confirming its superiority as a scaffold for drug delivery.
Keywords: Chitosan; X-ray sensitivity; affinity; cytotoxicity; inflammation; magnetic nanoparticle; uptake.
Copyright© 2020, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures





References
-
- Ducret M, Montembault A, Josse J, Pasdeloup M, Celle A, Benchrih R, Mallein-Gerin F, Alliot-Licht B, David L, Farges JC. Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent Mater. 2019;35(4):523–533. doi: 10.1016/j.dental.2019.01.018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
