Cardiomyocyte-specific deletion of GCN5L1 in mice restricts mitochondrial protein hyperacetylation in response to a high fat diet
- PMID: 32606301
- PMCID: PMC7326908
- DOI: 10.1038/s41598-020-67812-x
Cardiomyocyte-specific deletion of GCN5L1 in mice restricts mitochondrial protein hyperacetylation in response to a high fat diet
Abstract
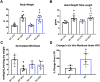
Mitochondrial lysine acetylation regulates several metabolic pathways in cardiac cells. The current study investigated whether GCN5L1-mediated lysine acetylation regulates cardiac mitochondrial metabolic proteins in response to a high fat diet (HFD). GCN5L1 cardiac-specific knockout (cKO) mice showed significantly reduced mitochondrial protein acetylation following a HFD relative to wildtype (WT) mice. GCN5L1 cKO mice did not display any decrease in ex vivo cardiac workload in response to a HFD. In contrast, ex vivo cardiac function in HFD-fed WT mice dropped ~ 50% relative to low fat diet (LFD) fed controls. The acetylation status of electron transport chain Complex I protein NDUFB8 was significantly increased in WT mice fed a HFD, but remained unchanged in GCN5L1 cKO mice relative to LFD controls. Finally, we observed that inhibitory acetylation of superoxide dismutase 2 (SOD2) at K122 was increased in WT (but not cKO mice) on a HFD. This correlated with significantly increased cardiac lipid peroxidation in HFD-fed WT mice relative to GCN5L1 cKO animals under the same conditions. We conclude that increased GCN5L1 expression in response to a HFD promotes increased lysine acetylation, and that this may play a role in the development of reactive oxygen species (ROS) damage caused by nutrient excess.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

