Hfm1 participates in Golgi-associated spindle assembly and division in mouse oocyte meiosis
- PMID: 32606310
- PMCID: PMC7327073
- DOI: 10.1038/s41419-020-2697-4
Hfm1 participates in Golgi-associated spindle assembly and division in mouse oocyte meiosis
Abstract
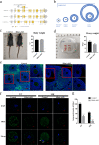
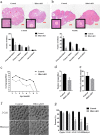
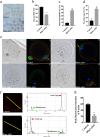
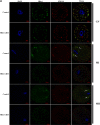
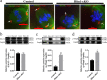
HFM1 (helicase for meiosis 1) is widely recognized as an ATP-dependent DNA helicase and is expressed mainly in germ-line cells. HFM1 is a candidate gene of premature ovarian failure (POF), hence it is also known as POF9. However, the roles of HFM1 in mammalian oocytes remain uncertain. To investigate the functions of HFM1, we established a conditional knockout (cKO) mouse model. Specific knockout of Hfm1 in mouse oocytes from the primordial follicle stage resulted in depletion of ovarian follicular reserve and subfertility of mice. In particular, abnormal spindle, misaligned chromosomes, loss of cortical actin cap, and failing polar body extrusion were readily observed in Hfm1-cKO oocytes. Further studies indicated that in addition to its cytoplasmic distribution, Hfm1 accumulated at the spindle poles, colocalized with the Golgi marker protein, GM130. Generally, GM130 signals overlapped with p-Mapk at the two spindle poles to regulate meiotic spindle assembly and asymmetric division. In this research, centrosome associated proteins, such as GM130 and p-Mapk, detached from the spindle poles in Hfm1-cKO oocytes. In conclusion, our data suggest that Hfm1 participates in Golgi-associated spindle assembly and division in mouse oocyte meiosis. These findings provide clues for pathogenesis of POF.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

