TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer
- PMID: 32606331
- PMCID: PMC7326979
- DOI: 10.1038/s41598-020-67325-7
TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer
Abstract
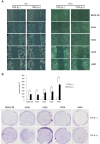
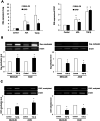
Transforming growth factor-β (TGF-β) promotes tumor invasion and metastasis by inducing epithelial-mesenchymal transition (EMT). EMT is often related with acquisition of stemness characteristics. The objective of this study was to determine whether EMT and stemness characteristics induced by TGF-β might be associated with epigenetic regulation in lung cancer. A human normal lung epithelial cell line and four lung cancer cell lines were treated with TGF-β. Transcriptome analysis of BEAS-2B and A549 cells incubated with TGF-β were analyzed through next-generation sequencing (NGS). Western blotting was carried out to investigate expression levels of epithelial and mesenchymal markers. Wound healing and Matrigel invasion assay, sphere formation assay, and in vivo mice tumor model were performed to evaluate functional characteristics of EMT and stemness acquisition. To investigate whether activation of EMT and stem cell markers might be involved in epigenetic regulation of lung cancer, experiment using a DNA methyltransferase inhibitor (5-azacytidine, AZA), methylation-specific PCR (MSP) and bisulfite sequencing were performed. NGS revealed changes in expression levels of EMT markers (E-cadherin, N-cadherin, fibronectin, vimentin, slug and snail) and stem cell markers (CD44 and CD87) in both BEAS-2B and A549 cells. Functional analysis revealed increased migration, invasion, sphere formation, and tumor development in mice after TGF-β treatment. Expression of slug and CD87 genes was activated following treatment with AZA and TGF-β. MSP and bisulfite sequencing indicated DNA demethylation of slug and CD87 genes. These results suggest that TGF-β induced EMT and cancer stemness acquisition could be associated with activation of slug and CD87 gene by their promoter demethylation.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

