Treatment Mode Preferences in Psoriatic Arthritis: A Qualitative Multi-Country Study
- PMID: 32606613
- PMCID: PMC7293411
- DOI: 10.2147/PPA.S242336
Treatment Mode Preferences in Psoriatic Arthritis: A Qualitative Multi-Country Study
Abstract
Objective: Qualitative research exploring patient preferences regarding the mode of treatment administration for psoriatic arthritis (PsA) is limited. We report patient preferences and their reasons across PsA treatment modes.
Methods: In this global, cross-sectional, qualitative study, interviews were conducted with adult patients with PsA in Brazil, France, Germany, Italy, Spain, the UK, and the US. Patients were currently taking a disease-modifying antirheumatic drug (DMARD). Patients indicated the order and strength of preference (0-100; 100 = strongest) across four modes of treatment administration: oral (once daily), self-injection (weekly), clinic injection (weekly), and infusion (monthly); reasons for preferences were qualitatively assessed. Descriptive statistics were reported. Fisher's exact tests and t-tests were conducted for treatment mode outcomes.
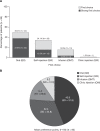
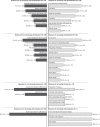
Results: Overall, 85 patients were interviewed (female, 60.0%; mean age, 49.8 years). First-choice ranking (%) and mean [standard deviation] preference points were: oral (49.4%; 43.9 [31.9]); self-injection (34.1%; 32.4 [24.8]); infusion (15.3%; 14.5 [20.0]); clinic injection (1.2%; 9.2 [10.0]). Of 48 (56.5%) patients with a strong first-choice preference (ie point allocation ≥60), 66.7% chose oral administration. Self-injection was most often selected as second choice (51.8%), clinic injection as third (49.4%), and infusion as fourth (47.1%). Oral administration was the first-choice preference in the US (88.0% vs 38.0% in Europe). The most commonly reported reason for oral administration as the first choice was speed and ease of administration (76.2%); for self-injection, this was convenience (75.9%). The most commonly reported reason for avoiding oral administration was concern about possible drug interactions (63.6%); for self-injection, this was a dislike of needles or the injection process (66.7%).
Conclusion: Patients with PsA preferred oral treatment administration, followed by self-injection; convenience factors were common reasons for these preferences. Overall, 43.5% of patients did not feel strongly about their first-choice preference and may benefit from discussions with healthcare professionals about PsA treatment administration options.
Keywords: patient preference; psoriatic arthritis; qualitative research; treatment administration.
© 2020 Aletaha et al.
Conflict of interest statement
DA has received grant/research support from AbbVie, Bristol-Myers Squibb, and MSD; is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Medac, Merck, MSD, Pfizer Inc, Roche, and UCB; and is a member of speakers’ bureaus for AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Medac, Merck, MSD, Pfizer Inc, Roche, and UCB. MEH is a consultant for and reports personal fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB. JFM is a consultant and/or investigator for and reports personal fees from AbbVie, Aclaris, Almirall, Arena, Avotres, Biogen, Celgene, Dermavant, Eli Lilly, GSK, Incyte, Janssen, Leo Pharma, Merck, Novartis, Pfizer Inc, EMD Serono, Samumed, Sanofi Regeneron, Sun Pharma, and UCB. RR is a consultant for AbbVie, Janssen, Novartis, and Pfizer Inc; and is a member of speakers’ bureaus for AbbVie, Janssen, Novartis, and Pfizer Inc. HB is a consultant for Pfizer Inc. RL was an employee and shareholder of Pfizer Inc at the time of analysis. RL reports personal fees from Pfizer Pharma GmbH, during the conduct of the study; personal fees from AbbVie Inc and Pfizer Pharma GmbH, outside the submitted work. TMB and CE are employees of RTI Health Solutions (non-profit independent research institute), which receives funding from pharmaceutical companies for research consulting services. TMB and CE receive no compensation from the pharmaceutical companies, and their RTI salary is not connected to the projects they work on or the clients for whom research is conducted. PMY, JCC, M-AH, and LF are employees of, and hold stock/stock options in, Pfizer Inc. The authors report no other conflicts of interest in this work.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

