Formulation and Characterization of Sertaconazole Nitrate Mucoadhesive Liposomes for Vaginal Candidiasis
- PMID: 32606665
- PMCID: PMC7295534
- DOI: 10.2147/IJN.S250960
Formulation and Characterization of Sertaconazole Nitrate Mucoadhesive Liposomes for Vaginal Candidiasis
Abstract
Purpose: The aim of this study is to develop efficient localized therapy of sertaconazole nitrate for the treatment of vaginal candidiasis.
Methods: Sertaconazole nitrate-loaded cationic liposomes were prepared by thin-film hydration method and coated with different concentrations of pectin (0.05%, 0.1% and 0.2%) to develop mucoadhesive liposomes. The formulated mucoadhesive vesicles were characterized in terms of morphology, entrapment efficiency, particle size, zeta value, mucoadhesive properties and drug release. The selected formula was incorporated into a gel base and further characterized by an ex vivo permeation study in comparison with conventional sertaconazole gel. Also, the in vivo study was performed to assess the efficacy of sertaconazole mucoadhesive liposomal gel in treating rats with vaginal candidiasis.
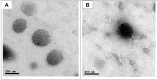
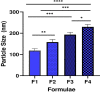
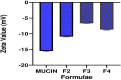
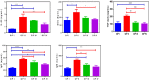
Results: The mucoadhesive liposomes were spherical. Coating liposomes with pectin results in increased entrapment efficiency and particle size compared with uncoated vesicles. On the contrary, zeta values were reduced upon coating liposomes with pectin indicating efficient coating of liposomes with pectin. Mucoadhesive liposomes showed a more prolonged and sustained drug release compared with uncoated liposomes. Ex vivo study results showed that mucoadhesive liposomal gel increased sertaconazole tissue retention and reduced drug tissue penetration. In the invivo study, the mucoadhesive liposomal gel showed a significant reduction in the microbial count with a subsequent reduction in inflammatory responses with the lowest histopathological change compared with conventional gel.
Conclusion: The study confirmed the potentiality of employing mucoadhesive liposomes as a successful carrier for the vaginal delivery of antifungal drugs.
Keywords: mucoadhesive liposomes; sertaconazole nitrate; vaginal candidiasis.
© 2020 Abdellatif et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
-
- Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807–815. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

