Safety and Efficacy of Centanafadine Sustained-Release in Adults With Attention-Deficit Hyperactivity Disorder: Results of Phase 2 Studies
- PMID: 32606695
- PMCID: PMC7292254
- DOI: 10.2147/NDT.S242084
Safety and Efficacy of Centanafadine Sustained-Release in Adults With Attention-Deficit Hyperactivity Disorder: Results of Phase 2 Studies
Abstract
Purpose: Two phase 2 studies evaluated the efficacy and tolerability of centanafadine sustained-release (SR) for adults with attention-deficit/hyperactivity disorder (ADHD).
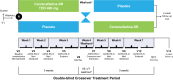
Patients and methods: In a phase 2a, flexible-dose, single-blind study, 41 male patients (aged 18‒55 years) with a diagnosis of ADHD (based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) were titrated with centanafadine-SR 200‒300, 400, or 500 mg/d for 2 weeks, and then were treated with the titrated dose for 2 weeks. In a phase 2b, randomized, double-blind, placebo-controlled, crossover study, 85 male and female patients (aged 18‒60 years) with a diagnosis of ADHD (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) were titrated to target doses of centanafadine-SR 400, 500, 600, or 800 mg/d over the course of 1 week, and then received their titrated dose for 3 weeks. The primary outcome in both studies was mean total ADHD Rating Scale-IV (ADHD-RS-IV) score.
Results: In the phase 2a study, mean ADHD-RS-IV total score decreased by 21.41 (standard deviation 10.74) from the start of active centanafadine-SR treatment to the end of week 4 (P<0.001). In the phase 2b study, centanafadine-SR treatment resulted in a statistically significant improvement in ADHD-RS-IV from baseline to week 3 compared with placebo (least-squares mean -16.5 vs -8.4; P<0.001; effect size 0.66), with significant efficacy demonstrated as early as week 1. Centanafadine-SR was generally well tolerated at doses ≤400 mg. Most treatment-emergent adverse events (TEAEs) were mild or moderate; decreased appetite, headache, and nausea were the most frequently reported. In the 2 studies, 13 of 120 patients discontinued centanafadine-SR due to TEAEs; however, only 1 patient who received ≤400 mg discontinued due to a TEAE. No serious TEAEs were reported at any dose.
Conclusion: These results support the continued development of centanafadine-SR at doses up to 400 mg/d.
Keywords: ADHD Rating Scale-IV; efficacy; norepinephrine-dopamine-serotonin reuptake inhibitor; tolerability.
© 2020 Wigal et al.
Conflict of interest statement
Dr Sharon B. Wigal reports grants from Neurovance and Otsuka during the conduct of the studies; consultant, advisory board, and/or speakers fees from Cingulate Therapeutics, Ironshore, Neurovance, NLS, Otsuka, Pfizer, Purdue, Rho, Rhodes, Sunovion and Supernus outside the submitted work. Dr Tim Wigal received consultant, advisory board, and/or research fees from Neurovance and Otsuka during the conduct of the studies; and from Cingulate Therapeutics, Ironshore, Neurovance, NLS, Otsuka, Pfizer, Purdue, Rhodes, Sunovion, and Supernus outside the submitted work. Drs Mary Hobart, Jessica J Madera, Ross A Baker, and Eva Kohegyi are full-time employees for Otsuka Pharmaceuticals Development & Commercialization, Inc. Mr Anthony McKinney was the President and CEO of Neurovance, and inventor of relevant patents. He received salary, bonus, and stock from Neurovanceand has patents 9856217, 9839627, and 9708261 issued. Dr Timothy E Wilens consulted for Otsuka during the conduct of the studies; Massachusetts General Hospital received consulting fees from Otsuka, Ironshore, KemPharm, and Vallon on behalf of work conducted by Dr Wilens; Dr Wilens received grants from NIH (NIDA), is co-owner of a copyrighted diagnostic questionnaire—Before School Functioning Questionnaire—with Ironshore, received royalties for the published book “Straight Talk About Psychiatric Medications for Kids” from Guilford Press and for co-editing the textbook “ADHD in Adults and Children” from Cambridge University Press, and received personal fees from Gavin Foundation, Bay Cove Human Services, US National Football League (ERM Associates), and US Minor/Major League Baseball outside the submitted work. The authors report no other conflicts of interest in this work.
Figures




References
-
- Cortese S, Adamo N, Mohr-Jensen C, et al. Comparative efficacy and tolerability of medications for attention deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psych. 2018;5(9):727–738. doi: 10.1016/S2215-0366(18)30269-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

