Sustained Corticosteroid-Free Clinical Remission During Vedolizumab Maintenance Therapy in Patients with Ulcerative Colitis on Stable Concomitant Corticosteroids During Induction Therapy: A Post Hoc Analysis of GEMINI 1
- PMID: 32606883
- PMCID: PMC7295209
- DOI: 10.2147/CEG.S248597
Sustained Corticosteroid-Free Clinical Remission During Vedolizumab Maintenance Therapy in Patients with Ulcerative Colitis on Stable Concomitant Corticosteroids During Induction Therapy: A Post Hoc Analysis of GEMINI 1
Abstract
Background: Corticosteroid-free clinical remission is important in ulcerative colitis.
Objective: This GEMINI 1 post hoc analysis evaluated vedolizumab efficacy in achieving sustained corticosteroid-free clinical remission in moderately to severely active ulcerative colitis.
Materials and methods: GEMINI 1 included a 6-week induction period followed by a 46-week maintenance period. Patients received stable corticosteroid dosing at baseline/during induction and tapered dosing during maintenance. Analysis groups included vedolizumab (induction and maintenance); vedolizumab/placebo (vedolizumab induction, placebo maintenance); and placebo (induction and maintenance). The primary endpoint was sustained corticosteroid-free clinical remission (partial Mayo score ≤2, no individual subscore >1, for ≥32 weeks). Multivariate analyses identified covariates associated with the primary endpoint. Safety endpoints included adverse events.
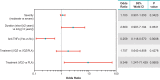
Results: Baseline demographics and concomitant corticosteroid use were similar across groups (n=454). A greater proportion (95% confidence interval) of the vedolizumab group achieved sustained corticosteroid-free clinical remission (10.2% [6.9 to 13.6]) vs the placebo group (1.4% [0.0 to 7.3]; difference 8.9% [-3.8 to 21.4]). Proportions were similar between the vedolizumab/placebo and placebo groups. Covariates associated with sustained corticosteroid-free clinical remission (odds ratio [95% confidence interval]) were treatment (vedolizumab vs placebo: 9.35 [1.25 to 71.43]; p=0.0605), anti-tumor necrosis factor alpha exposure (yes vs no: 0.26 [0.12 to 0.57]; p=0.0008), and disease duration (≤2 vs >2 years: 2.66 [0.99-7.19]; p=0.0531). Adverse events were similar across groups.
Conclusion: A numerically greater proportion of vedolizumab-treated patients with ulcerative colitis achieved sustained corticosteroid-free clinical remission. Vedolizumab treatment, no previous anti-tumor necrosis factor alpha exposure, and shorter disease duration were associated with sustained corticosteroid-free clinical remission.
Clinicaltrialsgov: NCT00783718.
Keywords: anti-tumor necrosis factor alpha; clinical remission; corticosteroid; ulcerative colitis; vedolizumab.
© 2020 Loftus Jr et al.
Conflict of interest statement
EVL has received financial support for research from: AbbVie, Takeda, Janssen, UCB, Amgen, Pfizer, Genentech, Celgene, Receptos, Gilead, MedImmune, Seres Therapeutics, and Robarts Clinical Trials; and has served as a consultant for AbbVie, Takeda, Janssen, UCB, Amgen, Pfizer, Eli Lilly, Celltrion Healthcare, Allergan, Bristol-Myers Squibb, Gilead, Genentech, Celgene, and Boehringer Ingelheim. BES has received financial support for research from: Takeda, Janssen, Theravance Biopharma, Pfizer; and has served as a consultant for: 4D Pharma, AbbVie, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Capella Bioscience, Celgene, Celltrion Healthcare, Eli Lilly and Company, EnGene, F. Hoffmann-La Roche, Ferring, Gilead Sciences, Ironwood Pharmaceuticals, Janssen, Lyndra, MedImmune, Oppilan Pharma, Otsuka America Pharmaceutical, Palatin Technologies, Pfizer, Progenity, Prometheus Laboratories, Protagonist Therapeutics, Rheos Medicines, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Synergy Pharmaceuticals, Takeda, Target PharmaSolutions, Theravance Biopharma, TiGenix, UCB, Valeant Pharmaceuticals North America, Vivelix Pharmaceuticals. J-FC has served as a consultant/advisory board member for: AbbVie, Amgen, Arena Pharmaceuticals, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Geneva Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Immunic, Ipsen, Landos, Medimmune, Merck & Co., Novartis, O Mass, Otsuka, Pfizer, Takeda, Tigenix, and Viela Bio; has served as a speaker for: AbbVie, Allergan, Amgen, Ferring Pharmaceuticals, and Takeda; has received research support from: AbbVie, Janssen Pharmaceuticals, and Takeda; and has stock options in: Intestinal Biotech Development and Genfit. ID has served as a consultant/advisory board member for: Pfizer, AbbVie, Janssen, Takeda, Genentech, Neopharm, Celltrion, Celgene, Arena, Gilead, Medtronic/given imaging, Rafa Laboratories; has served as a speaker for: Takeda, AbbVie, Janssen, Genentech, Pfizer, Ferring, Falk Pharma, Celltrion; has received research support from: Pfizer, AbbVie. JMK was an employee of Takeda International - UK Branch at the time this study was conducted. DT was a biostatistics consultant on temporary contract at Takeda Pharmaceuticals AG at the time this study was conducted. PG is an employee of Takeda Pharmaceuticals International AG and holds Takeda stock or stock options. The authors report no other conflicts of interest in this work.
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Medical

