Pharmacokinetics, Efficacy and Safety of a Plasma-Derived VWF/FVIII Concentrate (Formulation V) in Pediatric Patients with von Willebrand Disease (SWIFTLY-VWD Study)
- PMID: 32607039
- PMCID: PMC7319533
- DOI: 10.2147/JBM.S236789
Pharmacokinetics, Efficacy and Safety of a Plasma-Derived VWF/FVIII Concentrate (Formulation V) in Pediatric Patients with von Willebrand Disease (SWIFTLY-VWD Study)
Abstract
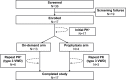
Purpose: Formulation V (VONCENTO®) is a plasma-derived high-concentration/low-volume, high-purity von Willebrand factor (VWF)/factor VIII (FVIII) concentrate, originally indicated for von Willebrand disease (VWD) in adults and adolescents. This multicenter, open-label study (SWIFTLY-VWD) evaluated the pharmacokinetics (PK), as well as hemostatic efficacy and safety, of Formulation V in pediatric patients (<12 years) with severe VWD requiring treatment or prophylaxis of bleedings.
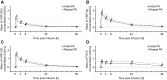
Methods: PK investigations were performed following one dose of Formulation V at Day 1 and 180. Nonsurgical bleeds were analyzed, while hemostatic efficacy was graded as excellent/good/moderate/none. Safety assessments included adverse events, and presence of VWF and/or FVIII inhibitors.
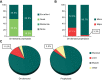
Results: Formulation V was administered as on-demand (N=13) or prophylaxis therapy (N=4) for 12 months (<6 years, N=9; 6 to <12 years, N=8). PK parameters for VWF markers were generally comparable to adults but showed lower VWF:ristocetin cofactor (RCo) exposure. Incidence of major bleeds was lower for prophylaxis (3.3%) than on-demand therapy (27.1%); joint bleeds were also lower (3.3% vs 11.5%, respectively). Investigator-reported excellent/good hemostatic efficacy against nonsurgical bleeds was 100%. No clinically relevant differences in PK, hemostatic efficacy, or safety were observed between age-groups (<6 years and 6 to <12 years). Formulation V was well tolerated. Adverse events were mild-moderate and consistent with the adult safety profile. No cases of anaphylactic reactions or angioedema, development of FVIII/VWF inhibitors, thromboembolic events, or viral infections were reported.
Conclusion: This study provides evidence for use of Formulation V to treat and prevent bleeding in pediatric patients with severe VWD, and led to the European approval of Formulation V in children.
Keywords: clinical trial; pediatrics; prophylaxis; von Willebrand disease; von Willebrand factor-factor VIII concentrate.
© 2020 Auerswald et al.
Conflict of interest statement
GA has received reimbursements for attending symposia/congresses and/or honoraria for speaking and/or consulting and/or funds for research support from CSL Behring. OS has received honoraria from Novo Nordisk, Takeda, and CSL Behring, and declares membership of speaker bureaus for Novo Nordisk, Takeda and Pfizer. WS and TR are employees of CSL Behring. The authors report no other conflicts of interest in this work.
Figures
References
-
- Flood VH, Montgomery RR. von Willebrand disease: biologic diagnosis In: Lee CA, Berntorp EE, Hoots WK, editors. Textbook of Hemophilia. Oxford, UK: Wiley-Blackwell; 2010:294–301.
LinkOut - more resources
Full Text Sources
Miscellaneous

