Real-World Results of Ocrelizumab Treatment for Primary Progressive Multiple Sclerosis
- PMID: 32607256
- PMCID: PMC7313157
- DOI: 10.1155/2020/5463451
Real-World Results of Ocrelizumab Treatment for Primary Progressive Multiple Sclerosis
Abstract
Background: Recently, ocrelizumab (Ocrevus®) was approved for the treatment of primary progressive multiple sclerosis (PPMS) based on data from the ORATORIO clinical trial. Real-world data about the clinical effectiveness of ocrelizumab has yet to be gathered.
Objective: The aim of this study was to provide data about the clinical effectiveness of ocrelizumab for patients diagnosed with PPMS in a real-world setting.
Methods: We conducted a retrospective cohort study of all patients with PPMS who started ocrelizumab treatment (n = 21) in St. Antonius Hospital (Utrecht/Nieuwegein, the Netherlands) between April 2018 and December 31, 2018. Primary outcome was pre- versus post-ocrelizumab disability worsening rate (from 96 weeks prior to first ocrelizumab administration up to 24 weeks post first ocrelizumab administration).
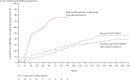
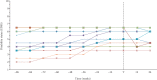
Results: Disability worsening rate while on treatment significantly differed (lower) from disability worsening rate in pre-treatment period (Z = -2.81, p ≤ .01). Three out of 17 patients showed a clinically relevant improvement in disability status after treatment start.
Conclusion: Ocrelizumab can stabilize disability progression in patients with PPMS. Some patients even showed a clinically relevant improvement in disability status. Further research should help to identify which patients benefit most from ocrelizumab.
Copyright © 2020 K. Daniels et al.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Food and Drug Administration. Use of real-world evidence to support regulatory decision-making for medical devices: draft guidance for industry and Food and Drug Administration staff. 2017. January 2019, https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance....
LinkOut - more resources
Full Text Sources
Miscellaneous

