This is a preprint.
Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance
- PMID: 32607516
- PMCID: PMC7325181
- DOI: 10.1101/2020.06.22.20136309
Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance
Update in
-
Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening.Sci Adv. 2021 Jan 1;7(1):eabd5393. doi: 10.1126/sciadv.abd5393. Print 2021 Jan. Sci Adv. 2021. PMID: 33219112 Free PMC article.
Abstract
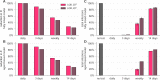
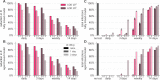
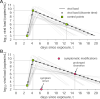
The COVID-19 pandemic has created a public health crisis. Because SARS-CoV-2 can spread from individuals with pre-symptomatic, symptomatic, and asymptomatic infections, the re-opening of societies and the control of virus spread will be facilitated by robust surveillance, for which virus testing will often be central. After infection, individuals undergo a period of incubation during which viral titers are usually too low to detect, followed by an exponential viral growth, leading to a peak viral load and infectiousness, and ending with declining viral levels and clearance. Given the pattern of viral load kinetics, we model surveillance effectiveness considering test sensitivities, frequency, and sample-to-answer reporting time. These results demonstrate that effective surveillance depends largely on frequency of testing and the speed of reporting, and is only marginally improved by high test sensitivity. We therefore conclude that surveillance should prioritize accessibility, frequency, and sample-to-answer time; analytical limits of detection should be secondary.
Figures



References
-
- Arons Melissa M, Hatfield Kelly M, Reddy Sujan C, Kimball Anne, James Allison, Jacobs Jesica R, Taylor Joanne, Spicer Kevin, Bardossy Ana C, Oakley Lisa P, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New England Journal of Medicine, 2020. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
