Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19
- PMID: 32607555
- PMCID: PMC7337789
- DOI: 10.1093/jac/dkaa239
Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19
Abstract
Background: Remdesivir is a prodrug of the nucleoside analogue GS-441524 and is under evaluation for treatment of SARS-CoV-2-infected patients.
Objectives: To evaluate the pharmacokinetics of remdesivir and GS-441524 in plasma, bronchoalveolar aspirate (BAS) and CSF in two critically ill COVID-19 patients.
Methods: Remdesivir was administered at 200 mg loading dose on the first day followed by 12 days of 100 mg in two critically ill patients. Blood samples were collected immediately after (C0) and at 1 (C1) and 24 h (C24) after intravenous administration on day 3 until day 9. BAS samples were collected on Days 4, 7 and 9 from both patients while one CSF on Day 7 was obtained in one patient. Remdesivir and GS-441524 concentrations were measured in these samples using a validated UHPLC-MS/MS method.
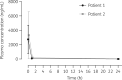
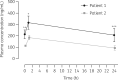
Results: We observed higher concentrations of remdesivir at C0 (6- to 7-fold higher than EC50 from in vitro studies) and a notable decay at C1. GS-441524 plasma concentrations reached a peak at C1 and persisted until the next administration. Higher concentrations of GS-441524 were observed in the patient with mild renal dysfunction. Mean BAS/plasma concentration ratios of GS-441524 were 2.3% and 6.4% in Patient 1 and Patient 2, respectively. The CSF concentration found in Patient 2 was 25.7% with respect to plasma. GS-441524 levels in lung and CNS suggest compartmental differences in drug exposure.
Conclusions: We report the first pharmacokinetic evaluation of remdesivir and GS-441524 in recovered COVID-19 patients. Further study of the pharmacokinetic profile of remdesivir, GS-441524 and the intracellular triphosphate form are required.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Li G, De Clercq E.. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020; 19: 149–50. - PubMed
-
- Siegel D, Hui HC, Doerrfler E. et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem 2017; 60: 1648–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

