Design and evaluation of bi-functional iron chelators for protection of dopaminergic neurons from toxicants
- PMID: 32607613
- PMCID: PMC7415766
- DOI: 10.1007/s00204-020-02826-y
Design and evaluation of bi-functional iron chelators for protection of dopaminergic neurons from toxicants
Abstract
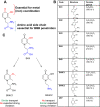
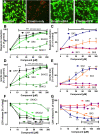
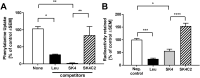
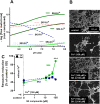
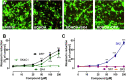
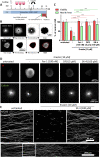
While the etiology of non-familial Parkinson's disease (PD) remains unclear, there is evidence that increased levels of tissue iron may be a contributing factor. Moreover, exposure to some environmental toxicants is considered an additional risk factor. Therefore, brain-targeted iron chelators are of interest as antidotes for poisoning with dopaminergic toxicants, and as potential treatment of PD. We, therefore, designed a series of small molecules with high affinity for ferric iron and containing structural elements to allow their transport to the brain via the neutral amino acid transporter, LAT1 (SLC7A5). Five candidate molecules were synthesized and initially characterized for protection from ferroptosis in human neurons. The promising hydroxypyridinone SK4 was characterized further. Selective iron chelation within the physiological range of pH values and uptake by LAT1 were confirmed. Concentrations of 10-20 µM blocked neurite loss and cell demise triggered by the parkinsonian neurotoxicants, methyl-phenyl-pyridinium (MPP+) and 6-hydroxydopamine (6-OHDA) in human dopaminergic neuronal cultures (LUHMES cells). Rescue was also observed when chelators were given after the toxicant. SK4 derivatives that either lacked LAT1 affinity or had reduced iron chelation potency showed altered activity in our assay panel, as expected. Thus, an iron chelator was developed that revealed neuroprotective properties, as assessed in several models. The data strongly support the role of iron in dopaminergic neurotoxicity and suggests further exploration of the proposed design strategy for improving brain iron chelation.
Keywords: Blood–brain barrier; Dopaminergic neurons; Drug design; Hydroxypyridinones; Iron chelators; LAT1; Parkinson’s disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Baes CF, Mesmer RE. The hydrolysis of cations. Krieger, Malabar, Fla: R.E; 1986.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

