Interactions between cannabidiol and Δ9 -tetrahydrocannabinol in modulating seizure susceptibility and survival in a mouse model of Dravet syndrome
- PMID: 32608111
- PMCID: PMC7443476
- DOI: 10.1111/bph.15181
Interactions between cannabidiol and Δ9 -tetrahydrocannabinol in modulating seizure susceptibility and survival in a mouse model of Dravet syndrome
Abstract
Background and purpose: Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome. These extracts typically contain cannabidiol (CBD), a phytocannabinoid with well-documented anticonvulsant effects, but may also contain Δ9 -tetrahydrocannabinol (Δ9 -THC). It is unclear whether the presence of Δ9 -THC modulates the anticonvulsant efficacy of CBD. Here, we utilized the Scn1a+/- mouse model of Dravet syndrome to examine this question.
Experimental approach: Scn1a+/- mice recapitulate core features of Dravet syndrome, including hyperthermia-induced seizures, early onset spontaneous seizures and sudden death. We assessed the effects on CBD and Δ9 -THC alone, and in combination on hyperthermia-induced seizures, spontaneous seizures and premature mortality.
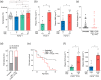
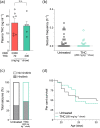
Key results: Administered alone, CBD (100 mg·kg-1 i.p.) was anticonvulsant against hyperthermia-induced seizures as were low (0.1 and 0.3 mg·kg-1 i.p.) but not higher doses of Δ9 -THC. A subthreshold dose of CBD (12 mg·kg-1 ) enhanced the anticonvulsant effects of Δ9 -THC (0.1 mg·kg-1 ). Sub-chronic oral administration of Δ9 -THC or CBD alone did not affect spontaneous seizure frequency or mortality while, surprisingly, their co-administration increased the severity of spontaneous seizures and overall mortality.
Conclusion and implications: Low doses of Δ9 -THC are anticonvulsant against hyperthermia-induced seizures in Scn1a+/- mice, effects that are enhanced by a sub-anticonvulsant dose of CBD. However, proconvulsant effects and increased premature mortality are observed when CBD and Δ9 -THC are sub-chronically dosed in combination. The possible explanations and implications of this are discussed.
© 2020 The British Pharmacological Society.
Conflict of interest statement
Associate Professor Jonathon Arnold is Deputy Academic Director of the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded research centre at the University of Sydney. He has served as an expert witness in various medicolegal cases involving cannabis and in 2018 was a temporary advisor to the World Health Organization (WHO) on their review of cannabis and the cannabinoids. His research is funded by the Lambert Initiative and the Australian National Health and Medical Research Council (NHMRC). A/Prof Arnold, Dr Anderson, and Prof McGregor hold patents on cannabinoid therapies (PCT/AU2018/05089 and PCT/AU2019/050554). Prof McGregor is Academic Director of the Lambert Initiative for Cannabinoid Therapeutics. He has served as an expert witness in various medicolegal cases involving cannabis use, has received honoraria from Janssen, is currently a consultant to Kinoxis Therapeutics, and has received research funding and fellowships from the NHMRC and Australian Research Council (ARC).
Figures




References
-
- Anderson, L. L. , Absalom, N. L. , Abelev, S. V. , Low, I. K. , Doohan, P. T. , Martin, L. J. , … Arnold, J. C. (2019). Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia, 60(11), 2224–2234. 10.1111/epi.16355 - DOI - PMC - PubMed
-
- Anderson, L. L. , Low, I. K. , Banister, S. D. , McGregor, I. S. , & Arnold, J. C. (2019). Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet syndrome. Journal of Natural Products, 82(11), 3047–3055. 10.1021/acs.jnatprod.9b00600 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

