Silk degumming time controls horseradish peroxidase-catalyzed hydrogel properties
- PMID: 32608410
- PMCID: PMC7390697
- DOI: 10.1039/d0bm00512f
Silk degumming time controls horseradish peroxidase-catalyzed hydrogel properties
Abstract
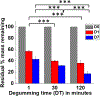
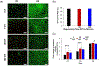
Hydrogels provide promising applications in tissue engineering and regenerative medicine, with silk fibroin (SF) offering biocompatibility, biodegradability and tunable mechanical properties. The molecular weight (MW) distribution of SF chains varies from ∼80 to 400 kDa depending on the extraction and purification process utilized to prepare the protein polymer. Here, we report a fundamental study on the effect of different silk degumming (extraction) time (DT) on biomaterial properties of enzymatically crosslinked hydrogels, including secondary structure, mechanical stiffness, in vitro degradation, swelling/contraction, optical transparency and cell behaviour. The results indicate that DT plays a crucial role in determining material properties of the hydrogel; decrease in DT increases β-sheet (crystal) formation and mechanical stiffness while decreasing degradation rate and optical transparency. The findings on the relationships between properties of silk hydrogels and DT should facilitate the more rational design of silk-based hydrogel biomaterials to match properties needed for diverse purpose in biomedical engineering.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interest.
Figures

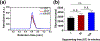


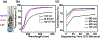
Similar articles
-
Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation.Biomaterials. 2020 Feb;232:119720. doi: 10.1016/j.biomaterials.2019.119720. Epub 2019 Dec 23. Biomaterials. 2020. PMID: 31896515 Free PMC article.
-
Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels.Acta Biomater. 2021 Apr 15;125:57-71. doi: 10.1016/j.actbio.2021.02.018. Epub 2021 Feb 16. Acta Biomater. 2021. PMID: 33601067 Review.
-
Enzymatically crosslinked and mechanically tunable silk fibroin/pullulan hydrogels for mesenchymal stem cells delivery.Int J Biol Macromol. 2018 Aug;115:300-307. doi: 10.1016/j.ijbiomac.2018.04.046. Epub 2018 Apr 14. Int J Biol Macromol. 2018. PMID: 29665386
-
Horseradish Peroxidase Catalyzed Silk-Prefoldin Composite Hydrogel Networks.ACS Appl Bio Mater. 2023 Jan 16;6(1):203-208. doi: 10.1021/acsabm.2c00836. Epub 2022 Dec 29. ACS Appl Bio Mater. 2023. PMID: 36580433
-
Silk protein-based hydrogels: Promising advanced materials for biomedical applications.Acta Biomater. 2016 Feb;31:17-32. doi: 10.1016/j.actbio.2015.11.034. Epub 2015 Nov 18. Acta Biomater. 2016. PMID: 26602821 Review.
Cited by
-
Molecular Engineering of Recombinant Protein Hydrogels: Programmable Design and Biomedical Applications.Gels. 2025 Jul 26;11(8):579. doi: 10.3390/gels11080579. Gels. 2025. PMID: 40868710 Free PMC article. Review.
-
Hydrogel for light delivery in biomedical applications.Bioact Mater. 2024 Apr 23;37:407-423. doi: 10.1016/j.bioactmat.2024.03.031. eCollection 2024 Jul. Bioact Mater. 2024. PMID: 38689660 Free PMC article. Review.
-
Synthesis and characterization of silk-poly(guluronate) hybrid polymers for the fabrication of dual crosslinked, mechanically dynamic hydrogels.Polymer (Guildf). 2023 Jul 18;281:126129. doi: 10.1016/j.polymer.2023.126129. Epub 2023 Jun 20. Polymer (Guildf). 2023. PMID: 37483847 Free PMC article.
-
3D-printed micrometer-scale wireless magnetic cilia with metachronal programmability.Sci Adv. 2023 Mar 22;9(12):eadf9462. doi: 10.1126/sciadv.adf9462. Epub 2023 Mar 22. Sci Adv. 2023. PMID: 36947622 Free PMC article.
-
Multifunctional silk vinyl sulfone-based hydrogel scaffolds for dynamic material-cell interactions.Biomaterials. 2023 Sep;300:122201. doi: 10.1016/j.biomaterials.2023.122201. Epub 2023 Jun 14. Biomaterials. 2023. PMID: 37348323 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

