Natural products as inspiration for the development of bacterial antibiofilm agents
- PMID: 32608431
- PMCID: PMC7677205
- DOI: 10.1039/d0np00022a
Natural products as inspiration for the development of bacterial antibiofilm agents
Abstract
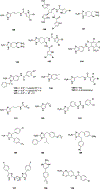
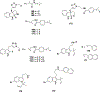
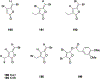
Natural products have historically been a rich source of diverse chemical matter with numerous biological activities, and have played an important role in drug discovery in many areas including infectious disease. Synthetic and medicinal chemistry have been, and continue to be, important tools to realize the potential of natural products as therapeutics and as chemical probes. The formation of biofilms by bacteria in an infection setting is a significant factor in the recalcitrance of many bacterial infections, conferring increased tolerance to many antibiotics and to the host immune response, and as yet there are no approved therapeutics for combatting biofilm-based bacterial infections. Small molecules that interfere with the ability of bacteria to form and maintain biofilms can overcome antibiotic tolerance conferred by the biofilm phenotype, and have the potential to form combination therapies with conventional antibiotics. Many natural products with anti-biofilm activity have been identified from plants, microbes, and marine life, including: elligic acid glycosides, hamamelitannin, carolacton, skyllamycins, promysalin, phenazines, bromoageliferin, flustramine C, meridianin D, and brominated furanones. Total synthesis and medicinal chemistry programs have facilitated structure confirmation, identification of critical structural motifs, better understanding of mechanistic pathways, and the development of more potent, more accessible, or more pharmacologically favorable derivatives of anti-biofilm natural products.
Conflict of interest statement
Conflicts of interest
Dr. C. Melander is co-founder of Agile Sciences, a biotechnology company seeking to commercialize anti-biofilm compounds.
Figures


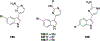


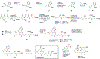

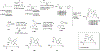


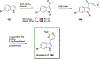
Similar articles
-
Leveraging Marine Natural Products as a Platform to Tackle Bacterial Resistance and Persistence.Acc Chem Res. 2021 Apr 20;54(8):1866-1877. doi: 10.1021/acs.accounts.1c00007. Epub 2021 Mar 18. Acc Chem Res. 2021. PMID: 33733746 Free PMC article.
-
Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms.Phytomedicine. 2017 Dec 15;37:14-26. doi: 10.1016/j.phymed.2017.10.021. Epub 2017 Nov 23. Phytomedicine. 2017. PMID: 29174600 Review.
-
Anti-Larval and Anti-Algal Natural Products from Marine Microorganisms as Sources of Anti-Biofilm Agents.Mar Drugs. 2022 Jan 21;20(2):90. doi: 10.3390/md20020090. Mar Drugs. 2022. PMID: 35200620 Free PMC article. Review.
-
Second-Generation Meridianin Analogues Inhibit the Formation of Mycobacterium smegmatis Biofilms and Sensitize Polymyxin-Resistant Gram-Negative Bacteria to Colistin.ChemMedChem. 2020 Sep 3;15(17):1672-1679. doi: 10.1002/cmdc.202000438. Epub 2020 Aug 3. ChemMedChem. 2020. PMID: 32662926 Free PMC article.
-
Natural Products as Platforms To Overcome Antibiotic Resistance.Chem Rev. 2017 Oct 11;117(19):12415-12474. doi: 10.1021/acs.chemrev.7b00283. Epub 2017 Sep 27. Chem Rev. 2017. PMID: 28953368 Free PMC article. Review.
Cited by
-
Innovative Strategies to Overcome Antimicrobial Resistance and Tolerance.Microorganisms. 2022 Dec 21;11(1):16. doi: 10.3390/microorganisms11010016. Microorganisms. 2022. PMID: 36677308 Free PMC article. Review.
-
Helicobacter pylori Biofilm-Related Drug Resistance and New Developments in Its Anti-Biofilm Agents.Infect Drug Resist. 2022 Apr 5;15:1561-1571. doi: 10.2147/IDR.S357473. eCollection 2022. Infect Drug Resist. 2022. PMID: 35411160 Free PMC article. Review.
-
A systematic review of astragaloside IV effects on animal models of diabetes mellitus and its complications.Heliyon. 2024 Feb 22;10(5):e26863. doi: 10.1016/j.heliyon.2024.e26863. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38439832 Free PMC article. Review.
-
Overcoming antibiotic-resistant Helicobacter pylori infection: Current challenges and emerging approaches.World J Gastroenterol. 2025 Mar 14;31(10):102289. doi: 10.3748/wjg.v31.i10.102289. World J Gastroenterol. 2025. PMID: 40093672 Free PMC article. Review.
-
Leveraging Marine Natural Products as a Platform to Tackle Bacterial Resistance and Persistence.Acc Chem Res. 2021 Apr 20;54(8):1866-1877. doi: 10.1021/acs.accounts.1c00007. Epub 2021 Mar 18. Acc Chem Res. 2021. PMID: 33733746 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

