Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis
- PMID: 32608556
- PMCID: PMC7445410
- DOI: 10.1111/cpr.12830
Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis
Abstract
Objectives: Skin serves as the major interface between the external environment and body which is liable to many kinds of injuries. Mesenchymal stem cell (MSC) therapy has been widely used and became a promising strategy. Pre-treatment with chemical agents, hypoxia or gene modifications can partially protect MSCs against injury, and the pre-treated MSCs show the improved differentiation, homing capacity, survival and paracrine effects regard to attenuating injury. The aim of this study was to investigate whether the exosomes from the educated MSCs contribute to accelerate wound healing process.
Materials and methods: We extracted the exosomes from the two educated MSCs and utilized them in the cutaneous wound healing model. The pro-angiogenetic effect of exosomes on endothelial cells was also investigated.
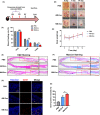
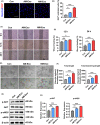
Results: We firstly found that MSCs pre-treated by exosomes from neonatal serum significantly improved their biological functions and the effect of therapy. Moreover, we extracted the exosomes from the educated MSCs and utilized them to treat the cutaneous wound model directly. We found that the released exosomes from MSCs which educated by neonatal serum before had the more outstanding performance in therapeutic effect. Mechanistically, we revealed that the recipient endothelial cells (ECs) were targeted and the exosomes promoted their functions to enhance angiogenesis via regulating AKT/eNOS pathway.
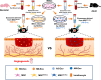
Conclusions: Our findings unravelled the positive effect of the upgraded exosomes from the educated MSCs as a promising cell-free therapeutic strategy for cutaneous wound healing.
Keywords: angiogenesis; exosome; mesenchymal stem cell; regenerative medicine; wound healing.
© 2020 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314‐321. - PubMed
-
- Qi Y, Jiang D, Sindrilaru A, et al. TSG‐6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full‐thickness skin wounds. J Invest Dermatol. 2014;134(2):526‐537. - PubMed
MeSH terms
Grants and funding
- 2018JM3026/Natural Science Basic Research Program of Shaanxi
- 2018D4/The Young Talent Support Program of Stomatology of FMMU
- 2017QNRC001/Young Elite Scientist Sponsorship Program by CAST
- 2016YFC1102900/National Key Research and Development Program of China
- 31800817/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

