Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma
- PMID: 32608579
- PMCID: PMC7433814
- DOI: 10.1002/cam4.3259
Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma
Abstract
Purpose: Anti-CD19 chimeric antigen receptor T (CAR-T) cell therapy has demonstrated remarkable efficacy for refractory and relapsed diffuse large B cell lymphoma (R/R DLBCL). However, this therapy failed in nearly 25% patients mainly due to antigen loss. The authors performed a phase Ⅱ trial by coadministration of anti-CD19 and anti-CD20 CAR-T cells treatment for R/R DLBCL and evaluated its efficacy and toxicity.
Methods: Totally 21 patients with DLBCL were enrolled in this study. The patients were conditioned with fludarabine and cyclophosphamide before the infusion of anti-CD19 and anti-CD20 CAR-T cells. Treatment response, toxicity, and persistence were monitored continuously.
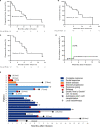
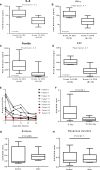
Results: Of the 21 patients received the treatment, the objective response rate (ORR) is 81.0% (95% confidence interval [CI], 58.1%-94.6%) with four cases of bulk (4/5) and one case of testis involvement; 52.4% (95% CI, 29.8%-74.3%) had a complete response (CR). Peak levels of anti-CD19 and anti-CD20 CAR cells were associated with response (P = .007 and .002). Grade 3-4 cytokine release syndrome (CRS) and neurologic events occurred in 28.5% and 9.5% patients, respectively. Median overall survival (OS) and progression-free survival (PFS) were 8.1 and 5.0 months, respectively. The maximum standard uptake value (SUVmax) of CD4/CD8 ratio before and after infusion were associated with responses, and the total lesion glycolysis (TLG) before infusion correlates with cytokines level.
Conclusions: Coadministration of anti-CD19 and CD20 CAR-T cells therapy for DLBCL is feasible with manageable toxicity. Cytokine markers are related to toxicity and SUVmax could predict efficacy. This trial was registered at www.clinicaltrials.gov as NCT03207178.
Keywords: CAR-T; CD19; CD20; DLBCL; clinical trial.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
No potential conflict of interest was disclosed.
Figures



References
-
- Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B‐cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027‐5033. - PubMed
-
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040‐2045. - PMC - PubMed
-
- Thakral B, Medeiros LJ, Desai P, et al. Prognostic impact of CD5 expression in diffuse large B‐cell lymphoma in patients treated with rituximab‐EPOCH. Eur J Haematol. 2017;98:415‐421. - PubMed
-
- Staiger AM, Ziepert M, Horn H, et al. Clinical impact of the cell‐of‐origin classification and the MYC/BCL2 dual expresser status in diffuse large B‐cell lymphoma treated within prospective clinical trials of the German High‐Grade Non‐Hodgkin's Lymphoma Study Group. J Clin Oncol. 2017;35:2515‐2526. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

