Biochemically Tracked Variability of Blood Plasma Thawed-State Exposure Times in a Multisite Collection Study
- PMID: 32608993
- PMCID: PMC9836705
- DOI: 10.1089/bio.2019.0137
Biochemically Tracked Variability of Blood Plasma Thawed-State Exposure Times in a Multisite Collection Study
Abstract
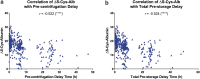
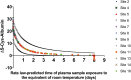
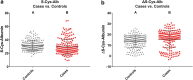
The integrity of blood plasma/serum (P/S) specimens can be impacted by preanalytical handling and storage conditions that result in thawed-state exposures (> -30°C). We recently reported a simple dilute-and-shoot, intact-protein liquid chromatography/mass spectrometry (LC/MS) assay called ΔS-Cys-Albumin that quantifies cumulative exposure of P/S to thawed conditions based on the change in relative abundance of the oxidized (S-cysteinylated) proteoform of albumin (S-Cys-Albumin) in the native sample to that of an aliquot of the sample intentionally driven to its maximum oxidation state. Herein, we evaluated the effect of prestorage delay and initial storage temperature on sample integrity by applying the ΔS-Cys-Albumin assay to a set of plasma samples (n = 413) collected under a single clinical study but from 12 different collection sites. Major differences (p < 0.0001) were observed between different groups of samples with modestly inconsistent initial handling conditions (i.e., initial processing of whole blood to plasma and placement at -80°C completed in under 3 hours, 3-13 hours, and over 17 hours). ΔS-Cys-Albumin was significantly inversely correlated with delay time at 4°C before centrifugation and total delay before final storage at -80°C (p < 0.0001). Samples from two collection sites had much lower ΔS-Cys-Albumin values relative to samples from other sites, in accordance with the fact that they were stored at -20°C for an average of 7.6 months before shipment to the central repository for final storage at -80°C. Based on the rate law for S-Cys-Albumin formation in plasma ex vivo, the average time that each plasma specimen had been exposed to the equivalent of room temperature (23°C) was back calculated from the measured ΔS-Cys-Albumin values. A survey of clinical analytes in P/S whose measured concentrations are sensitive to the initial handling/storage conditions documented in this study is provided and the ramifications of the plasma integrity findings from this multisite clinical study are discussed.
Keywords: WELCA; albumin; biospecimen integrity; plasma; thawed; ΔS-Cys-Albumin.
Conflict of interest statement
No conflicting financial interests exist.
Figures



References
-
- Poste G, Compton CC, Barker AD. The national biomarker development alliance: Confronting the poor productivity of biomarker research and development. Expert Rev Mol Diagn 2015;15:211–218. - PubMed
-
- Ellervik C, Vaught J. Preanalytical variables affecting the integrity of human biospecimens in biobanking. Clin Chem 2015;61:914–934. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

